Crystal-size Distributions and Possible Biogenic Origin of ...
University of Zurich - UZHformed during the metabolism of vitamins, steroids, lipids, amino acids,...
Transcript of University of Zurich - UZHformed during the metabolism of vitamins, steroids, lipids, amino acids,...

University of ZurichZurich Open Repository and Archive
Winterthurerstr. 190
CH-8057 Zurich
http://www.zora.uzh.ch
Year: 2010
Increased Activity of Mitochondrial Aldehyde Dehydrogenase(ALDH) in the Putamen of Individuals with Alzheimer's
Disease: A Human Postmortem Study
Michel, T M; Gsell, W; Käsbauer, L; Tatschner, T; Sheldrick, A J; Neuner, I;Schneider, F; Grünblatt, E; Riederer, P
Michel, T M; Gsell, W; Käsbauer, L; Tatschner, T; Sheldrick, A J; Neuner, I; Schneider, F; Grünblatt, E; Riederer,P (2010). Increased Activity of Mitochondrial Aldehyde Dehydrogenase (ALDH) in the Putamen of Individualswith Alzheimer's Disease: A Human Postmortem Study. Journal of Alzheimer's Disease:Epub ahead of print.Postprint available at:http://www.zora.uzh.ch
Posted at the Zurich Open Repository and Archive, University of Zurich.http://www.zora.uzh.ch
Originally published at:Journal of Alzheimer's Disease 2010, :Epub ahead of print.
Michel, T M; Gsell, W; Käsbauer, L; Tatschner, T; Sheldrick, A J; Neuner, I; Schneider, F; Grünblatt, E; Riederer,P (2010). Increased Activity of Mitochondrial Aldehyde Dehydrogenase (ALDH) in the Putamen of Individualswith Alzheimer's Disease: A Human Postmortem Study. Journal of Alzheimer's Disease:Epub ahead of print.Postprint available at:http://www.zora.uzh.ch
Posted at the Zurich Open Repository and Archive, University of Zurich.http://www.zora.uzh.ch
Originally published at:Journal of Alzheimer's Disease 2010, :Epub ahead of print.

Increased Activity of Mitochondrial Aldehyde Dehydrogenase(ALDH) in the Putamen of Individuals with Alzheimer's
Disease: A Human Postmortem Study
Abstract
For decades, it has been acknowledged that oxidative stress due to free radical species contributes to thepathophysiology of aging and neurodegenerative diseases. Aldehyde dehydrogenases (ALDH) not onlytransform aldehydes to acids but also act as antioxidant enzymes. However, little is known about theimplications of the enzymatic family of ALDH in the context of neurodegenerative processes such asAlzheimer's disease (AD). We therefore examined the enzymatic activity of the mitochondrialALDH-isoform in different regions of the postmortem brain tissue isolated from patients with AD andcontrols. We found that the mitochondrial ALDH activity was significantly increased only in theputamen of patients suffering from AD compared to controls. This is of particular interest sincemediators of oxidative stress, such as iron, are increased in the putamen of patients with AD. This studyadds to the body of evidence that suggests that oxidative stress as well as aldehyde toxicity play a role inAD.

1
Journal of Alzheimer’s Disease ISSN: 1387-2877 IOS Press DOI: 10.3233/JAD-2009-1326
Increased Activity of Mitochondrial Aldehyde Dehydrogenase (ALDH) in the Putamen of
Individuals with Alzheimer’s Disease: A Human Postmortem Study
Tanja Maria Michela,b,c, Wieland Gselld, Ludwig Käsbauerb, Thomas Tatschnere, Abigail Jane
Sheldricka, Irene Neunera, Frank Schneidera,c, Edna Grünblattb and Peter Riederera
aDepartment of Psychiatry and Psychotherapy, RWTH Aachen University, Aachen, Germany
bClinical Neurochemistry, University Clinic & Policlinic Psychiatry, Psychosomatic and
Psychotherapy, University of Würzburg, Würzburg, Germany
cJARA – Translational Brain Medicine, Aachen, Germany
dState Hospital for Psychiatry, Psychotherapy and Neurology, Lohr am Main, Germany
eDepartment of Neuropathology, Institute of Pathology, University of Würzburg, Germany
Running title: Mitochondrial ALDH in Alzheimer’s disease
Accepted 1 November 2009
Correspondence to: Tanja Maria Michel, MD, Department of Psychiatry and Psychotherapy,
RWTH Aachen University, Pauwelsstraße 30, 52074 Aachen, Germany; Tel:+492418036258,
Fax:+492418082524, Email: tmichel@ukaachen.

2
ABSTRACT
For decades, it has been acknowledged that oxidative stress due to free radical species
contributes to the pathophysiology of aging and neurodegenerative diseases. Aldehyde
dehydrogenases (ALDH) not only transform aldehydes to acids but also act as antioxidant
enzymes. However, little is known about the implications of the enzymatic family of ALDH in
the context of neurodegenerative processes such as Alzheimer’s disease (AD). We therefore
examined the enzymatic activity of the mitochondrial ALDH-isoform in different regions of the
postmortem brain tissue isolated from patients with AD and controls. We found that the
mitochondrial ALDH activity was significantly increased only in the putamen of patients
suffering from AD compared to controls. This is of particular interest since mediators of
oxidative stress, such as iron, are increased in the putamen of patients with AD. This study adds
to the body of evidence that suggests that oxidative stress as well as aldehyde toxicity play a role
in AD.
Keywords: aging, aldehydehydrogenase, Alzheimer’s disease, dementia, free radicals,
mitochondria, oxidative stress

3
INTRODUCTION
In Alzheimer’s disease (AD), increased toxic damage to the brain due to increased reactive
aldehydes (RA) has been associated with neuropathological hallmarks of the disease such as
neurofibrillary tangles and neuritic plaques [1]. RA are highly reactive transient molecules,
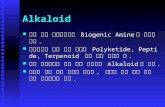
which are induced by endogenous and exogenous processes (Figure 1a-c). If not properly
degraded, RA target cell membranes and induce lipid peroxidation, modify enzyme function,
inducing mitochondrial dysfunction, and induce oxidative stress, which is also mediated by
increased cerebral iron, leading to apoptosis [2]. Both oxidative stress and aldehyde toxicity have
been acknowledged to play a key role in the pathophysiology of neuropsychiatric disorders and
AD via lipid peroxidation and DNA strand brakes [3-12]. Oxidative stress refers to an imbalance
within cells, where more reactive oxygen species (ROS) are produced than are detoxified,
leading to cell damage and death [13,14].
One of the most important pathways for aldehyde detoxification is their oxidation by
aldehyde dehydrogenase (ALDH, [EC 1.2.1.3.]). ALDH is a well known enzyme with pyridine-
nucleotide-dependent oxidoreductase activity which is important for the metabolism of aldehyde
substrates in numerous species [15].
The ALDH superfamily includes NAD(P)+-dependent enzymes catalyzing the oxidation of a
wide spectrum of aliphatic and aromatic aldehyde substrates generated from various endogenous
and exogenous precursors to their corresponding carboxylic acids. Endogenous aldehydes are
formed during the metabolism of vitamins, steroids, lipids, amino acids, neurotroansmitters,
biogenic amines and in the process of lipid peroxidation (Figures 1b,c). Exogenous aldehydes are
formed during the metabolism of numerous agents such as alcohol, drugs and environmental
agents (Figure 1a).

4
In AD, one special focus has been on one of the isoenzymes, the mitochondrial ALDH
(ALDH2). This isoenzyme of ALDH is located in the mitochondrial matrix and is considered to
be principally responsible for the oxidation of acetaldehyde which is produced during different
endogenous and exogenous processes mentioned above. It is involved in the metabolism of
biogenic aldehydes produced by monoamines and oxidase-catalyzed deamination of
catecholamines and indoleamines [15-17]. Furthermore, dicarbonyl compounds such as
methylglyoxal or glyoxal are also substrates for ALDH. These compounds have been implicated
in the pathogenesis of AD [18,19].
A functional polymorphism of the mitochondrial aldehyde dehydrogenase gene (ALDH2 1/2
polymorphism) can influence the accumulation of acetaldehyde which may play a role in
Alzheimer’s disease (AD) and has a high prevalence among Mongoloids [20-22]. The results of
the association studies were varying among different Asian subpopulations. An association with
late-onset AD (LOAD), interacting synergistically with the presence of the apolipoprotein E
allele 4 (APOE ε4) has been detected in Japanese and Chinese subjects, whilst it was not
reported in a population of Koreans [20-24]. A longitudinal study found that the incidence of AD
in healthy participants was not correlated with ALDH polymorphism within 2.4 years [24]. In the
mouse model, it has been shown that a deficiency in mitochondrial ALDH2 leads to age
dependent memory impairment and increased lipid peroxidation [25]. Furthermore, these animals
show the same neuropathology, namely hyperphosphorylation as well as plaques and tangles, as
seen in humans with AD [25].
It has been suggested that mitochondrial ALDH influences the pathophysiology of AD in
several ways. First of all, it influences aldehyde toxicity directly, secondly it significantly
contributes the overall antioxidative potential on a cellular level, and thirdly it interferes with

5
various neurotransmitters that are decreased in the context of AD. All these different
mechanisms are well known to play a pivotal role in AD. However, to this date, mitochondrial
ALDH is not one of the well-studied antioxidant players that scientists expected to find fighting
free-radical damage. Little is known about the activity and function of this enzyme in the brain
of patients with AD [26]. For this reason, it is worth examining the activity of mitochondrial
ALDH more closely in this context. We have therefore studied the enzyme activity of
mitochondrial ALDH in the cerebral cortex as well as the putamen of patients with AD since
both regions are known to play a role in AD and have an increased iron concentration, which
mediates oxidative stress in both aging and AD [27-29].
MATERIALS AND METHODS
Brain tissue samples
The activity of the mitochondrial aldehydedehydrogenase, ALDH, in the human brain was
measured in postmortem brain tissue of 14 elderly patients with mostly final stages of AD (9
females, 5 males, mean age: 81.6±4.4 years) and 12 age-matched controls (8 females, 4 males,
mean age: 80.8±4.4 years) without any medical history of psychiatric or neurological diseases.
Two of the age-matched controls showed neurofibrillary tangles and senile plaques. The patients
fulfilled the diagnostic criteria of the International classifications of disease for AD [30].
Additionally, the diagnosis was verified via postmortem examination for senile plaques and
neurofibrillary tangles [31]. None of the brains examined had signs of other neurodegenerative
diseases such as Parkinson’s disease (PD). The brain regions investigated were the frontal cortex
and the putamen. Brain samples from Caucasian German speaking patients were matched with

6
control samples according to age, gender, and postmortem delay (See Tables 1, 2, and 3 for
details).
The postmortem brain tissue was collected via autopsies performed at the Department of
Psychiatry, Hospital, Mauer, Austria and at the Institute of Forensic Medicine, University of
Würzburg, Germany. All procedures were in accordance with the NIH Guide for the Care and
Use of Laboratory human tissue and were approved by the Ethics committee of the University of
Würzburg, Germany and were also in accordance with the declaration of Helsinki.
Tissue extraction
The brain tissue was obtained according to a standardized procedure [32]. The left
hemisphere was freshly frozen at -80°C. For further neuropathological investigations, the right
hemisphere was kept in formalin. Freshly frozen brain extracts were prepared by homogenization
in 10 volumes (w/v) of 0.25 M saccharine with a Polytron homogenizer at 1000 rpm followed by
sonification (Branson). After this procedure, centrifugation (600g for 10 min) was carried out for
10 min at 4°C. The cellular debris was then re-suspended with 0.25M sucrose and the
supernatant was centrifuged for another 10 min (15000g at 4°C; Sorvall RC-5C). The cellular
debris was then re-suspended again with 0.25M saccharine and then sonified. The supernatants
were centrifuged for another 60 min (100,000g, 4°C Beckmann L3-50). The sediment with the
mitochondrial fraction was re-suspended with 0.25M.

7
Protein quantification
For protein quantification of the brain homogenate, the protein assay kit provided by BioRad
on the basis of the method previously described [33] was used. Colorimetric visualization was
carried out at 595 nm.
Mitochondrial ALDH Activity
The mitochondrial ALDH activity (nmol/min/mg protein) was determined using a
colorimetric method modified according to the protocol described before [34]. Briefly, 50 mM
sodiumpyrophosphate-buffer (pH 8.8), 0.5 mM NAD+, 1 mM EDTA, 10 mM 2-
Mercaptoethanol, 2 µM Rotenon (1% of total volume), 0.5 % Triton X-100 and freshly prepared
homogenate were used as reactant and the substrate indol-3-acetaldehyde (IAA) was added to
start the reaction. 1 mg of the brain homogenate and 0.4 mg of the mitochondrial fraction were
added to the reagent-substrate mixture. We later omitted the mitochondrial fraction to calculate
the result for the cytosolic fraction (data not shown). The change in optical density at 340 nm
(37°C) was measured over time (20 min) by a spectrophotometer (552S UV/VIS; Perkin-Elmer,
USA) to obtain the ALDH activity (Lambert-Beer law). The activity was calculated by the PC
connected to the photometer using both the standard curve and the extinction coefficient
(Lambert-Beer law).
Statistics
We compared the different ALDH activities between the index-group (patients with AD) and
age matched control-group using student’s t-test on the statistical software graph-pad-prism
version 4.

8
The influence of independent variables such as gender, age, and postmortem delay was tested
using covariance analysis (ANCOVA). In addition, we examined the relation of age and ALDH
activity by performing Pearson Product Moment Correlation. Significance was set at p-value <
0.05.
RESULTS
The mitochondrial ALDH activity (mean±SEM in mmol/min/mg protein) was significantly
(p<0.029, df=22) elevated in the putamen of the AD group (7.60±0.54, 22) compared to the
control group (5.9±0.45) (See Figure 2). In the frontal cortex, we were not able to detect any
significant differences between the groups (AD: 8.29±0.49; controls: 8.07±0.43, p >0.74, df=24).
The effect size for the putamen was 0.2 and for the frontal cortex 0.13.
Although patients and controls were carefully pair-matched, we examined the influences of
confounding parameters such as age, gender, medication, and postmortem delay on ALDH
activities. There was no significant correlation with postmortem time (0.41<rho<0.36;
0.19<p<0.96) nor age (-0.49<rho<0.52; 0.11<p<0.94) in any of the investigated brain regions.
DISCUSSION
In our study, we found that mitochondrial ALDH-activity was significantly higher in the
putamen of patients with AD. Our results are in line with previous studies suggesting a role of
ALDH in the pathogenesis of AD [20-23,26].
Our results of an alteration of the mitochondrial ALDH activity in the brain of patients with
AD are supported by earlier findings of Picklo and colleagues [26] who also found the ALDH
activity to be significantly elevated in the brain of patients with AD. This group reported a

9
significant increase of ALDH in the temporal cortex of patients with AD in a significantly
smaller sample of only four patients with AD [26]. To the best of our knowledge, we are the first
to describe an increase of mitochondrial ALDH in the putamen of a patient with AD. This study
suggested that increased mitochondrial ALDH expression might be due to increased highly
reactive intermediates or pro-oxidants in AD, since mitochondria are one of the main sources of
free radicals in organs [26]. Earlier studies on ALDH in AD have suggested that increased
ALDH activity, as we found in the putamen, might play a main role in the formation of senile
plaques [26].
A number of reasons could be responsible for the increased mitochondrial ALDH activity
that we report in the putamen of patients with AD. First, it might be a result of increased
production of aldehydes due to an increase of monoamine oxidase B (MAO-B), which has been
reported in the context of AD [35]. Furthermore, the increased ALDH activity, together with an
elevated MAO-B activity, could be responsible for a higher dopamine turnover and an increased
oxidative stress which has been found in the putamen of patients with AD [35-37]. The latter
induces MAO-B activity, via a positive feed-back loop and in turn increases oxidative stress and
neurodegeneration [37,38].
Second, in the context of AD, serotonergic, dopaminergic, and noradrenergic neurons
degenerat, due to multiple factors, e.g., disruption in neurotrophic factors, and increased iron
mediated oxidative stress [37,39,40]. These neurodegenerative processes warrant an increase of
the metabolizing enzymes, e.g., aldehyde dehydrogenases AD [41].
Thirdly, it has been reported that the putamen in particular contains large quantities of iron
which increase with age [29]. Furthermore, in AD, iron deposits in the putamen have been
reported to be significantly elevated in subjects with cognitive deficits [27,28]. Since iron plays a

10
pivotal role in oxidative stress, increased iron content in the putamen of patients with AD could
contribute to elevated oxidative stress, which in turn could increase both ROS and RAs. Both
could then lead to neurodegeneration which is described in the context of AD [30,39,42]. The
increase of RA might warrant metabolism catalyzed by increased mitochondrial ALDH [41].
However, we did not measure the iron content of the putamen in our study.
Finally, several studies suggest that alcohol intake affects the development of AD [43],
because ethanol and its metabolite, acetaldehyde, are directly neurotoxic. Therefore, a
dysregulation of ALDH leading to a disruption of the ethanol mechanism resulting in increased
aldehyde toxicity might contribute the development of AD in the context of increased alcohol
intake (ADH) [43]. However, since none of our AD subjects had a significant history of alcohol
addiction, external ethanol intake might play only a minor role in the increase of mitochondrial
ALDH that we found in the putamen of the index group.
Similar to what we observed with ALDH, increased expression of enzymes to detoxify ROS
have been reported earlier in AD. One example is manganese superoxide dismutase (Mn-SOD)
which is localized in the mitochondria and catalyzes the detoxification of superoxide [36].
We did not find a significant correlation with either the postmortem time or age and ALDH
activity. This is suggestive of a high postmortem stability of the enzyme as has been reported
before [44].
Our study is the first to report a significantly increased mitochondrial ALDH activity in the
putamen of patients with AD. However, the role of neuroprotection and degeneration in AD
remains complex since neuroprotective agents such as ALDH, neurotrophic factors, and their
interaction with oxidative stress in AD play an important role and have to be examined in further
studies [39,40,45].

11
The significance of our current examinations is highlighted by a recent study showing that
treatment with methylene blue (methylthioninium chloride) may postpone or reverse
neurodegeneration in AD [46]. Methylene blue inhibits tau aggregation and works as a hydrogen
ion scavenger, an inhibitor of nitric oxide synthase as well as an inhibitor of the enzyme MAO
[47]. Both pathomechanisms are linked to oxidative stress as well as aldehyde toxicity and the
function of ALDH. Therefore, the therapeutic effect of methylene blue may be linked to its
ability to reduce oxidative stress and or aldehyde toxicity.
It is pivotal to carry out further studies linking possible mutations of the ALDH genes and
treatment outcome in AD. This has been carried out previously for other diseases that are linked
to ALDH and lead to neuronal damage or loss such as alcohol dependence [48]. Thus, the
metabolism of aldehyde, including the key enzyme mitochondrial ALDH, could be a new target
for preventive and therapeutic strategies in AD. However, so far, there are only few studies on
the subject and further investigations are needed for a better understanding of the complicated
interactions of this family of enzymes, namely ALDH, to explore future therapeutic implications.
DISCLOSURE STATEMENT
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=195).
REFERENCES
[1] Alonso A, Zaidi C, Novak T, Grundke-Iqbal I, Iqbal K (2001) Hyperphosphorylation
induces self-assembly of tau into tangles of paired helical filaments/straight filaments.
Proc Natl Acad Sci U S A 98, 6923-6928.

12
[2] Haber F, Weiss J (1934) The catalytic decomposition of hydrogen peroxide by iron salts.
Proc Roy Soc 147, 332-351.
[3] Reed TT, Pierce WM, Markesbery WR, Butterfield DA (2009) Proteomic identification
of HNE-bound proteins in early Alzheimer disease: Insight into the role of lipid
peroxidation in the progression of AD. Brain Res 1274, 66-76.
[4] Picklo MJ, Montine TJ (2007) Mitochondrial effects of lipid-derived neurotroxins. J
Alzheimers Dis 12, 185-193.
[5] Casado A, Encarnacion Lopez-Fernandez M, Concepcion Casado M, de la Torre R
(2008) Lipid peroxidation and antioxidant enzyme activities in vascular and Alzheimer
dementias. Neurochem Res 33, 450-458.
[6] Sofic E, Sapcanin A, Tahirovic I, Gavrankapetanovic I, Jellinger K, Reynolds GP,
Tatschner T, Riederer P (2006) Antioxidant capacity in postmortem brain tissues of
Parkinson's and Alzheimer's diseases. J Neural Transm Suppl 71, 39-43.
[7] Chen K, Kazachkov M, Yu PH (2007) Effect of aldehydes derived from oxidative
damination and oxidative stress on beta-amyloid aggregation; pathological implications
to Alzheimer’s disease. J Neural Transm 114, 835-839.
[8] Durany N, Muench G, Michel T, Riederer P (1999) Investigations on oxidative stress and
therapeutical imlications in dementia. Eur Arch Psychiatry Clin Neurosci (Germ) 249
Suppl 3, 68-73.
[9] Michel TM, Thome J, Nara K, Martin D, Camara S, Weijers HG, Riederer P, Koutsilieri
E (2004) The role of oxidative stress in the pathogenesis of schizophrenic psychoses -
Superoxide Dismutase (SOD)-coenzyme con-centrations in post-mortem brain tissue of
schizophrenic patients. J Neural Transm 111, 1191-1201.

13
[10] Ohta S, Ohsawa I (2006) Dysfunction of mitochondria and oxidative stress in the
pathogenesis of Alzheimer's disease: on defects in the cytochrome c oxidase complex and
aldehyde detoxification. J Alzheimers Dis 9, 155-166.
[11] Michel TM, Frangou S, Camara S, Tatschner T, Sheldrick AJ, Riederer P, Grünblatt E
(2008) Evidence for increased oxidative stress in the thalamus and in the putamen in
patients with recurrent depressive disorder – a post-mortem investigation. World J Biol
Psychiatry 12, 1-7.
[12] Michel TM, Frangou S, Thiemeyer D, Camara S, Zoechling R, Jecel J, Nara K,
Brunklaus A, Riederer P (2007) Evidence for oxidative stress in the frontal cortex in
patients with recurrent depressive disorder - a post-mortem study. Psychiatry Res 15,
145-150.
[13] Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and
antioxidants in normal physiological functions and human disease. Int J Biochem Cell
Biol 39, 44-84.
[14] Valko M, Morris H, Cronin MT (2005) Metals, toxicity and oxidative stress. Curr Med
Chem 12, 1161-1208.
[15] Ambroziak W, Pietruszko R (1991) Human aldehyde dehydrogenase activity with
aldehyde metabolites of monoamines, diamines and polyamines. J Biol Chem 266,
13011-13018.
[16] Forte-McRobbie CM, Pietruszko R (1986) Purification and characterization of human
liver "high Km" aldehyde dehydrogenase and its identification as glutamic gamma-
semialdehyde dehydrogenase. J Biol Chem 261, 2154-2163.

14
[17] Lindahl R, Evces S (1984) Rat liver aldehyde dehydrogenase. I. Isolation and
haracterization of four high Km normal liver isoenzymes. J Biol Chem 259, 11986-
11990.
[18] Kuhla B, Haase C, Flach K, Lüth HJ, Arendt T, Münch G (2007) Effect of
pseudophosphorylation and cross-linking by lipid peroxidation and advanced glycation
end product precursors on tau aggregation and filament formation. J Biol Chem 282,
6984-6991.
[19] Kuhla B, Boeck K, Schmidt A, Ogunlade V, Arendt T, Münch G, Lüth HJ (2006) Age-
and stage-dependent glyoxalase I expression and its activity in normal and Alzheimer's
disease brains. Neurobiol Aging 28, 29-41.
[20] Kamino K, Nagasaka K, Imagawa M, Yamamoto H, Yoneda H, Ueki A, Kitamura S,
Namekata K, Miki T, Ohta S (2000) Deficiency in mitochondrial aldehyde
dehydrogenase increases the risk for late-onset Alzheimer’s disease in the Japanese
population. Biochem Biophys Res Commun 273, 192-196.
[21] Ohta S, Ohsawa I, Kamino K, Ando F, Shimokata H (2004) Mitochondrial ALDH2
deficiency as an oxidative stress. Ann N Y Acad Sci 1011, 36-44.
[22] Wang B, Wang J, Zhou S, tan S, He X, Yang Z, Xie YC, Li S, Zheng C, Ma X (2008)
The association of mitochondrial aldehyde dehydrogenase gene (ALDH2) polymorphism
with susceptibility to late-onset Alzheimer’s disease in Chinese. J Neurol Sci 268, 172-
175.
[23] Wangad B, Wangad J, Zhouad S, Tanad S, Head X, Yangb Z, Xiec YZ, Lib S, Zhengf C,
Maade X (2008) The association of mitochondrial aldehyde dehydrogenase gene

15
(ALDH2) polymorphism with susceptibility to late-onset Alzheimer's disease in Chinese.
J Neural Sci 268, 172-175.
[24] Shin IS, Stewart R, Kim JM, Kim SW, Yang SJ, Shin HY, Jung JS, Yoon JS (2005)
Mitochondrial aldehyde dehydrogenase polymorphism is not associated with incidence of
Alzheimer’s disease. Int J Geriatr Psychiatry 20, 1075-1080.
[25] Ohsawa I, Nishimaki K, Murakami Y, Suzuki Y, Ishikawa M, Ohta S (2008) Age-
dependent neurodegeneration accompanying memory loss in transgenic mice defective in
mitochondrial aldehyde dehydrogenase 2 activity. J Neurosci 28, 6239-6249.
[26] Picklo MJ, Olson SJ, Markesbery WR, Montine TJ (2001) Expression and activities of
aldo-keto oxidoreductases in Alzheimer disease. J Neuropathol Exp Neurol 60, 686-695.
[27] Bartzokis G, Tishler TA (2000) MRI evaluation of basal ganglia ferritin iron and
neurotoxicity in Alzheimer's and Huntingon's disease. Cell Mol Biol (Noisy-le-grand) 46,
821-833.
[28] Bartzokis G, Sultzer D, Cummings J, Holt LE, Hance DB, Henderson VW, Mintz J
(2000) In vivo evaluation of brain iron in Alzheimer disease using magnetic resonance
imaging. Arch Gen Psychiatry 57, 47-53.
[29] Aquino D, Bizzi A, Grisoli M, Garavaglia B, Bruzzone MG, Nardocci N, Savoiardo M,
Chiapparini L (2009) Age related iron deposition in the basal ganglia: quantitative
analysis in healthy subjects. Radiology 252, 165-172.
[30] WHO (1992) ICD 10, International classification of diseases, Geneva, Switzerland.
[31] Tierney MC (1988), Fisher RH, Lewis AJ, Zorzitto ML, Snow WG, Reid DW,
Nieuwstaten P The NINCDS-ADRA work group criteria for the clinical diagnosis of
probable Alzheimer`s disease: clinicopathlogic study of 57 cases. Neurology 38, 359-364.

16
[32] Gsell W, Lange KW, Pfeuffer R, Heckers S, Heinsen H, Senitz D (1993) How to run a
brain bank. A report from the Austro-German brain bank. J Neural Transm Suppl 39, 31-
70.
[33] Bradford M (1976) A rapid and sensitive method for the quantification of microgram
quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72, 248-
254.
[34] Ryzlak MT, Pietruszko R (1987) Purification and characterization of aldehyde
dehydrogenase from human brain. Arch Biochem Biophys 255, 409-418.
[35] Arai H, Kosaka K, Iizuka R (1984) Changes of biogenic amines and their metabolites in
postmortem brains from patients with Alzheimer-type dementia. J Neurochem 17, 338-
393.
[36] Marklund Sl, Adolfsson R, Gottfries CG, Winblad G (1985) Superoxide dismutase
isoenzymes in normal brains and in brains from patients with dementia of the Alzheimer
type. J Neurol Sci 67, 319-325.
[37] Konradi C, Riederer P, Youdim MBH (1986) Hydrogen peroxide enhances the activity of
monoamine oxidase type B and not type A. A pilot study. J Neural Transm Suppl 22, 61-
73.
[38] Grünblatt E, Schlösser R, Fischer p, Fischer MO, Li J, Koutsilierei E, Wichart I, Stermba
N, Rujescu D, Möller HJ, Adamcyk W, Dittrich B, Müller F, Oberegger K, Gatterer G,
Jellinger KJ, Mostafaie N, Jungwirth S, Huber K, Tragl KH, Danielczyk W, Riederer P
(2005) Oxidative stress related markers in the “VITA” and the centenarian projects.
Neurobiol Aging 26, 429-438.

17
[39] Durany N, Michel T, Jellinger K, Cruz-Sanchez FF, Cervos-Navarro J, Riederer P (2000)
Brain-derived neurotrophic factor and neurotrophin-3 levels in Alzheimer’s disease
brains. Int J Devl Neurosci 18, 807-810.
[40] Michel TM, Nara K, Camara S, Koutsilieri E, Jecel JC, Riederer P (2004) Activity of
Xanthine Oxidase in different brain regions of patients with Alzheimer`s dementia – a
post mortem study. World J Biol Psych 5 Suppl 6212, 86.
[41] Balcz B, Kirchner L, Cairns N, Fountoulakis M, Lubec G (2001) Increased brain protein
levels of carbonyl reductase and alcohol dehydrogenase in Down syndrome and
Alzheimer's disease. J Neural Transm Suppl 61, 193-201.
[42] Furuta A, Price DL, Pardo CA (1995) Localization of superoxide dismutases in
Alzheimer’s disease and Down’s syndrome neuocortex and hippocampus. Am J Pathol
146, 357-356.
[43] Pietruszko R, Reed DM, Vallari RC, Major LF, Saini N, Hawley RJ (1981) Brain
aldehyde dehydrogenase in human alcoholics and controls. Alcoholism Clin Exp Res 5,
78-84.
[44] Fowler CJ, Wiberg A, Oreland L, Marusson J, Winblad B (1980) The effect of age on the
activity and molecular properties of human brain monoamine oxidase. J Neural Transm
49, 1-20.
[45] Thome J, Nara K, Foley P, Michel T, Gsell W, Retz W, Rösler M, Riederer P (1997)
CNTF genotypes: influence on choline acetyltransferase (ChAT) and acetylcholine
esterase (AChE) activities and neurotrophin 3 (NT3) concentration in human post
mortem brain tissue. J Hirnforsch 38, 443-451.

18
[46] Wischik CM (2008) O3-04-07: Tau aggregation inhibitor (TAI) therapy with remberTM
arrests disease progression in mild and moderate Alzheimer's disease over 50 weeks.
Alzheimers Dement 4 Suppl 1, T167.
[47] Gillman PK (2008) Methylene blue is a potent monoamine oxidase inhibitor. Can J
Anaesth 55, 311-312.
[48] Wiesbeck GA, Weijers HG, Wodarz N, Hermann M, Johann M, Keller H, Michel TM,
Böning J (2003) Dopamin D 2 (DAD 2) and dopamin D 3 (DAD 3) receptor gene
polymorphismus and treatment outcome in alcohol dependence. J Neural Transm 110,
813-820.

19
Table 1. Sample characteristics and activity of ALDH in the frontal cortex and the putamen. Number Age Gender
F/M Postmortem Cortex
frontalis p Putamen p
Control 12 80.8±7.5 8/4 8.2±5.1 8.07±0.43 5.9±0.45 AD 14 81.6 ± 4.4 9/5 9.4±9.5 8.29±0.49
>0.7 7.60±0.54
<0.03

20
Table 2. Demographic and neuropathological data of patients with AD Diagnosis Age Sex PM Cause of death Neuropathology/
Stage of disease AD 81 F 18 Pneumonia
Cardiac arrest Cerebral atrophy, Final stage of AD
AD 82 M 12 Pneumonia Cardiac arrest
Final stage AD
AD 83 M 6 Pulmonary embolism Cerebral Atrophy, internal hydrocephalus Final stage AD
AD 80 F 15 Sepsis Cerebral Atrophy Final stage AD
AD 80 F 4.5 Pulmonary embolism Cerebral Atrophy Final stage AD
AD 72 F 3 Sepsis
Cerebral Atrophy, micro-vascular cerebral infarction,
AD AD 86 F 38 Pulmonary embolism Diffuse cerebral Atrophy
AD (NS) AD 86 M 9 Pneumonia Diffuse cerebral Atrophy
AD (NS) AD 77 M 3.5 Cardiac arrest Atrophy
Cerebral arteriosclerosis AD
AD 88 F 3 Pulmonary embolism Diffuse cerebral Atrophy Final stage AD
AD 84 F 5 Cardiac arrest Diffuse cerebral Atrophy Final stage AD
AD 76 M 6 Pneumonia, Cardiac
arrest Cerebral Atrophy Final stage AD
AD 84 F 5 Pneumonia, Cardiac arrest
Cerebral Atrophy Final stage AD
AD 83 F 3 Ileus Cerebral Atrophy Final stage AD

21
Table 3. Demographic and neuropathological data of controls. Group Age Sex PM Cause of death Neuropathology
C 80 F 3.00 Pulmonary embolism - C 87 F 15.00 Cardiopmopathy Regular aged brain with a few
fibrillary tangles C 78 F 12.00 Pulmonary embolism
Cardiac arrest -
C 88 M 10.50 Pneumonia, Pulmonary embolism
-
C 72 F 3.00 Pulmonary embolism - C 85 F 13.00 Cardiac arrest - C 80 M 5.10 Acute cardial
dilatation, stroke Regular aged brain with a few
fibrillary tangles C 90 M 17.00 Cardiac arrest,
Pneumonia -
C 86 F 5.50 Pulmonary embolism - C 63 F 6.50 Bronchial carcinoma,
metastasis -
C 80 F 4.00 NK - C 80 M 3.50 Cardiac arrest
Sepsis -

22
FIGURE LEGEND
Figure 1. (a,b,c) The metabolism of ALDH, an exogenous ethanol-metabolism; (b)
neurotransmitter metabolism; (c) ALDH is detoxifying toxic aldehydes in the lipid peroxidation
process.

23
Figure 1