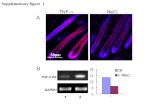
Supplementary Figure 1
-
Upload
tanner-delacruz -
Category
Documents
-
view
25 -
download
3
description
Transcript of Supplementary Figure 1

Supplementary Figure 1
0 (negative) 1 (weak) 2 (moderate) 3 (strong)
E F G H
1 (1 ~ 25%) 2 (26 ~ 50%) 3 (51 ~ 75%) 4 (76 ~ 100%)
A B C D

si negative si ANGPTL2 0
1
2
3
4
5
6
AN
GPTL2
/GA
PD
H
CA
Caco2 DLD1 HT29 Lovo SW4800
2
4
6
8
10
12
14
16
18
AN
GPTL2
/GA
PD
H
si negative si ANGPTL2 0
0.5
1
1.5
2
2.5
3
3.5
4
AN
GPTL2
/GA
PD
H
D
si negative si ANGPTL20
0.20.40.60.8
11.21.41.61.8
2
AN
GPTL2
(O
D)
E
AN
GPTL2
(O
D)
Caco2 DLD1 HT29 Lovo SW4800.45
0.50.55
0.60.65
0.70.75
0.80.85
0.9
B
si negative si ANGPTL20
0.5
1
1.5
2
2.5
AN
GPTL2
(O
D)
F
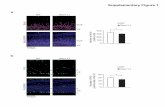
SW480
SW480
HT29
HT29
Supplementary Figure 2

Supplementary Figure 3
0 20 40 60 80 1000
20
40
60
80
100
100-Specificity
Sen
sitiv
ity
AUC = 0.859
CRC patients with CEA <5A
0 20 40 60 80 100
0
20
40
60
80
100
100-SpecificityS
ensi
tivity
Stage I CRC patients with CEA <5
AUC = 0.804
B