Strain Energy
description
Transcript of Strain Energy

Strain energyFrom Wikipedia, the free encyclopedia
In physics, strain energy is the energy stored by a system undergoing deformation. When the load is removed, strain energy as:U = \frac 1 2 V \sigma \epsilon = \frac 1 2 V E \epsilon^2 = \frac 1 2 \frac V E \sigma^2where σ is stress, ε is strain, V is volume, and E is Young's modulus:E = \frac \sigma \epsilon
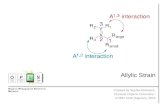
Molecular Strain
In a molecule, strain energy is released when the constituent atoms are allowed to rearrange themselves in a chemical reactioninterference are reduced.[1] The external work done on an elastic member in causing it to distort from its unstressed state is transformedinto strain energy which is a form of potential energy. The strain energy in the form of elastic deformation is mostly recoverable in theform of mechanical work.
For example, the heat of combustion of cyclopropane (696 kJ/mol) is higher than that of propane (657 kJ/mol) for each additional CH2unit. Compounds with unusually large strain energy include tetrahedranes, propellanes, cubanes, fenestranes and cyclophanes.
ReferencesMarch's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, Michael B. Smith & Jerry March, Wiley-Interscience, 5th edition,2001, ISBN 0-471-58589-0
1.
Retrieved from "https://en.wikipedia.org/w/index.php?title=Strain_energy&oldid=690489804"
Categories: Chemical bonding
This page was last modified on 13 November 2015, at 18:38.Text is available under the Creative Commons Attribution-ShareAlike License; additional terms may apply. By using this site, youagree to the Terms of Use and Privacy Policy. Wikipedia® is a registered trademark of the Wikimedia Foundation, Inc., a non-profitorganization.
Strain energy - Wikipedia, the free encyclopedia https://en.wikipedia.org/wiki/Strain_energy
نم 1 1 ص 06:42 29/11/2015PDF created with pdfFactory Pro trial version www.pdffactory.com




![Strain Transformation.ppt [相容模式] - 義守大學 · Plane-Strain Transformation Strain at the same point but different coordinates 6. Strain Transformation 4 want to calculate](https://static.fdocument.pub/doc/165x107/5b939ddc09d3f2d1448dc03d/strain-plane-strain-transformation-strain-at-the.jpg)