Intermediate filament assembly: dynamics to disease filament assembly: dynamics to disease Lisa M....
Transcript of Intermediate filament assembly: dynamics to disease filament assembly: dynamics to disease Lisa M....
Intermediate filament assembly:dynamics to diseaseLisa M. Godsel, Ryan P. Hobbs and Kathleen J. Green
Department of Pathology, Northwestern University Feinberg School of Medicine, 303 E. Chicago Avenue, Chicago IL 60611, USA
Review
Intermediate filament (IF) proteins belong to a large anddiverse gene family with broad representation invertebrate tissues. Although considered the ‘toughest’cytoskeletal fibers, studies in cultured cells have revealedthat IF can be surprisingly dynamic and highly regulated.This review examines the diversity of IF assembly beha-viors, and considers the ideas that IF proteins are co- orpost-translationally assembled into oligomeric precur-sors, which can be delivered to different subcellular com-partments by microtubules or actomyosin and associatedmotor proteins. Their interaction with other cellularelements via IF associated proteins (IFAPs) affects IFdynamics and also results in cellular networks with prop-erties that transcend those of individual components. Weend by discussing how mutations leading to defects in IFassembly, network formation or IF–IFAP association com-promise in vivo functions of IF as protectors againstenvironmental stress.
IntroductionIntermediate filaments (IF) are flexible, rod-shaped fibersaveraging�10 nm in diameter, a size that is ‘intermediate’between microfilaments (MF; 7–8 nm) and microtubules(MT; 25 nm) [1,2]. Of the three non-muscle cytoskeletalfibers, IFs are the most diverse and are encoded by anestimated 70 IF genes in the human genome (HumanIntermediate Filament Mutation Database; http://www.interfil.org). IFs are classified into five major familiesexpressed in cell-, tissue-, differentiation- and developmen-tal-specific patterns (Table 1). Families I–IV are localizedto the cell cytoplasmwhereas the type V nuclear lamins areimportant organizers of the nuclear envelope and karyo-plasm. IF family members share a common blueprint builtfrom a central a-helical coiled-coil rod flanked by flexible,highly variable N- and C-termini that lead to exceptionalstructural diversity among IFs [3]. This diversity presentsmany opportunities for tailoring IF networks to cell type-specific functions in contrast to the broadly conservedfunctions of MT and MF.
In most vertebrate cells cytoplasmic IFs are tetheredto the nucleus and extend into the cytoplasm where theyprovide a scaffold for mitochondria, the Golgi complex,microtubule organizing centers (MTOCs) and other cyto-skeletal elements (Figure 1) [1,2,4,5]. In the peripheryIFs associate with plasma membrane specializationssuch as desmosomes, hemidesmosomes and focal adhe-sions. The resulting network integrates and organizes the
Corresponding author: Godsel, L.M. ([email protected]).
28 0962-8924/$
cytoplasm providing mechanical integrity that is cru-cially important for tissue function. This is highlightedby a growing list of >75 human genetic diseases causedby deficiencies in this network, including skin fragilityand epidermolytic disorders, laminopathies, myopathies,neuropathies, cataracts and premature aging [6,7](Human Intermediate Filament Mutation Database,http://www.interfil.org). Notably, an emerging set ofmutations in nuclear lamins (reviewed elsewhere) com-prise a large proportion of human diseases attributable toIFs [8,9]. This article focuses on cytoplasmic IFs, whichare now recognized as players in cell signaling, growth,epithelial polarity, wound healing and apoptosis inaddition to providing the cell with resilience to environ-mental stress [2,10].
These broad-ranging functions derive from the diversityof IFs coupled with their unique mechanical and bio-chemical properties. IFs are the most flexible of the bio-logical filaments. Furthermore, unlike MFs and MTs, asingle IF can withstand stretching to more than threetimes its resting length before breaking [11]. Althoughintegration with other filament systems is necessary tocreate the final viscoelastic properties of the cytoplasm, itis thought that IFs contribute the tensile strength necess-ary for maintaining cell integrity (Box 1) [1,11,12]. IFs arealso biologically stable structures. However, a recent con-vergence of in vitro and in vivo explorations has shown theIF cytoskeleton to be a malleable and dynamic system thatcan be structurally and functionally tailored to suit cells’changing needs. In this review we explore how recenttrends have shaped our understanding of IF function,organization and assembly properties in the test tubeand in living cells. We have yet to fully understand howthese properties are translated into physiologicallyrelevant in vivo situations. However, the work discussedhere provides insight into how human disease phenotypesmight arise from fundamental defects in IF assembly andintegration with IF-associated proteins (IFAPs) into func-tionally competent networks.
IF structure and in vitro assembly propertiesIF proteins exhibit an extended secondary structure builtfrom a conserved a-helical rod domain of �310–350 aminoacids flanked by divergent non-helical N- and C-termini(Figure 2) [1]. The rod domain drives the formation ofparallel a-helical coiled-coil dimers through long-rangeheptad repeats organized as shown in Figure 2, each witha characteristic pattern of apolar residues in the first (a)and fourth (d) positions. These dimers constitute the
– see front matter � 2007 Elsevier Ltd. All rights reserved. doi:10.1016/j.tcb.2007.11.004
Table 1. Members of the IF superfamily
Type Cell types Localization Proteins Disease associations
I Epithelia Cytoplasm, hair Acidic keratins (pI < 5.7) K14 – epidermolysis bullosa simplex diseases
17 human epithelial keratins; K9–K28 K10, K16, K14 – keratoderma disorders
Hair 11 human hair keratins; K31–40 (Ha1–8) K12 – Meesmann corneal dystrophy
K13 – white sponge nevus of cannon
K16 – pachyonychia congenita type I
K17 – pachyonychia congenita type II
II Epithelia Cytoplasm, hair Basic keratins (pI � 6.0) K5 – epidermolysis bullosa simplex diseases
20 human epithelial keratins; K1–8, K71–80 K1, K9, K2 – keratoderma disorders
6 human hair keratins: K81–86 (Hb1–6) K3 – Meesmann corneal dystrophy
K4 – white sponge nevus of cannon
K6a – pachyonychia congenita type I
K6b – pachyonychia congenita type II
Hair K81 (Hb1), K83 (Hb3), K86 (Hb6) – monilethrix
K85 (Hb5) – pure hair-nail type ectodermal dysplasia
III Muscle Cytoplasm Desmin Desmin – desmin related myopathy, dilated
cardiomyopathy 1I, familial restrictive
cardiomyopathy 2
Mesenchymal Vimentin
Neurons Peripherin Peripherin – amyotrophic lateral sclerosis
Astrocytes and
glia
GFAP GFAP – Alexander disease
IV Neurons Cytoplasm NF-L NF-L, M and H – amyotrophic lateral sclerosis
Neurons NF-M NF-L – Charcot-Marie-Tooth diseases
Neurons NF-H NF-M – Parkinson disease
Neurons a-internexin NF-H – neuronal IF inclusion disease
Muscle Synemin a
Muscle Synemin b (desmuslin)
Muscle Syncoilin
Neuroepithelia Nestin
V Ubiquitous Nuclear lamina Lamins A/C Lamins – large number of disorders, including
lipodystrophies, muscular dystrophies, neurological
disorders and premature aging
B1
B2
Orphan Eye lens Cytoplasm Phakinin (CP49) CP49 – autosomal dominant cataract disease
Filensin (CP115) CP115 – autosomal recessive cataract disease
Review TRENDS in Cell Biology Vol.18 No.1
elemental building blocks of IFs and depending on the IFtype these can be hetero- (e.g. type I and II keratins andtype IV neurofilament chains) or homodimeric (e.g. type IIIvimentin and desmin).
A hypotheticalmodel based on the in vitro behavior of thetype III vimentin protein provides a useful platform forunderstanding how individual polypeptides might beassembled into an apolar filament (Figure 2). Accordingto this model, filament assembly comprises several majorsteps starting with the formation of parallel, in-registerdimers. Dimers then associate into tetramers, thought tobe organized primarily in a mode termed A11, in which the1B subunits of the rods overlap in an anti-parallel manner[13,14]. Tetramers aggregate into higher order oligomers toform unit length filaments (ULF) �60 nm long, whichundergo reorganization and elongation by longitudinalannealing to form immature IF. Within the polymer, otheranti-parallel arrangements A12, A22 and ACN can also occur,corresponding to associations between the 1B and 2B sub-domains, between the 2B subdomains, or between theC- and N-termini. The final step is radial compaction ofthe filament from �16 nm to a diameter of 10–12 nm. Thisstep has been proposed to occur via lateral rearrangementsof protein subunits such that compaction occurs withoutlosingmass or increasing the length of the filament [1,3,13].
The rod domains form the IF core and the N- andC-termini are displayed on the filament surface. It is
predicted that the head domains of type I and type IIepidermal keratins and type III IFs exhibit a flexiblestructure and could interact with sites in the rod domain.In both cases these N-termini are required for in vitrofilament assembly [13,15–17]. Deletion of the vimentinC-terminus did not block filament formation, but did resultin an increase in their mass-per-length [3]. However,mutations affecting the K5 tail found in patients withepidermolysis bullosa simplex (EBS) dramaticallyimpaired IF assembly and network formation [18]. Impor-tantly, exposure of the N- and C-termini on the filamentsurface also leaves them free to associate with other fila-ments and cellular structures.
We are far fromunderstanding the specificmechanism ofassembly for all IF family members, but it is clear thatdifferences exist, underscoredby the observed rapidkineticsof keratin polymerization and the completely distinctassembly behavior of nuclear lamins, which form longhead-to-tail arrays that associate laterally [16]. However,IF family members all share certain attributes that dis-tinguish them from MTs and MFs, including their apolarstructure, their inability to bind or hydrolyze nucleotidesand their capacity for undergoing cycles of assembly anddisassembly simply by altering the ionic strength and pH ofsolution [1,16]. By contrast, MFs and MTs are polar fila-ments that use cofactors to facilitate polymerization andconformational changes, as well as nucleotide hydrolysis to
29
Figure 1. IFs are integrators of cyto- and tissue architecture. IFs emanate from the nucleus, extending throughout the cytoplasm and out to contact points at the plasma
membrane. (a) Immunofluorescence microscopy reveals the keratin network in epithelial cells (red) linking to the desmosome (colocalization shown in yellow) via
interactions with the desmosomal plakin protein, desmoplakin (green). In this way the IF networks of adjacent cells are linked together giving the tissue mechanical
Review TRENDS in Cell Biology Vol.18 No.1
30
Figure 2. Schematic model of filament formation from dimer to mature filament, based on in vitro assembly data for the type III IF protein vimentin. Intermediate filaments
are made up of an a-helical coiled-coil domain flanked by two non-helical N- and C-termini (which vary considerably among IF types). IF assembly begins with the formation
of parallel a-helical coiled-coil dimers mediated by four heptad repeat-containing regions designated 1A, 1B, 2A and 2B, separated by short, flexible non-coiled linkers, L1,
L12 and L2 [1,3,17]. 1B and 2B harbor motifs important for ‘triggering’ and stabilizing the coiled-coil structure and for keratin IF heterodimerization [91]. Local variations in
the heptad repeat regions, such as a three residue deletion in 2B, also contribute to IF structure and assembly [17]. Dimers associate laterally into anti-parallel, half-
staggered tetramers or protofilaments in which the 1B subdomains are aligned – shown as a cylinder in this Figure [13]. ULFs subsequently anneal longitudinally to form
filaments that are loosely arranged and larger in caliber than mature filaments. During a subsequent compaction phase, IF are compressed radially from �16 nm to �10 nm
resulting in formation of a mature filament [1,3,13]. One hypothesis based on data acquired from vimentin studies suggests that the strands form a cylinder with a visible
open or low-density central core when observed in cross-section [3,13]. This figure presents a general model; however the number of tetramers varies when filaments are
viewed in cross-section and the extent of variation is dependent on IF type and assembly conditions [3,16].
Review TRENDS in Cell Biology Vol.18 No.1
regulate polymer dynamics. IF have not traditionally beenthought to require co-factors. However, recently it has beenshown that a neurofilament light chain (NF-L)-bindingprotein called NUDEL (nuclear distribution element-like),which regulates neuronal migration, facilitates assembly ofIF. This observation raises the possibility that there mightbe a more important role for co-factors in IF function thanoriginally thought [19].
IF dynamics in cells: initiation and remodeling ofIF networksAlthough IF polypeptides typically have long half-lives andare biochemically stable, IF networks routinely undergorearrangements involving disassembly and reassemblyduring processes such as cell spreading, wound healingand cell division, and in response to environmental stres-ses such as stretching and shear flow [20–28]. As describedhere, progress is being made in elucidating the rules that
strength. Scale bar, 20 mm. (b) The details of IF interactions with the nucleus, intracellul
nesprin and plakin family members to the nucleus, and extend into the cytoplasm whe
cyclodeaminase (FTCD)], and the endolysosomal system [e.g. adaptor protein complex
perhaps through interactions with the g-tubulin ring complex (gTuRC) protein GCP6
membrane proteins in polarized epithelial cells [5].
regulate polymerization of IF in vitro, but how these rulesapply to IF assembly and remodeling in cells remainslargely unknown.
One characteristic that is maintained in cells is theapolar nature of IFs and it has been speculated that IFsmight turn over or remodel in part through a process ofintrafilament exchange. The possibility that IF turnovercan occur by exchange within a network has been con-sidered for a number of years, based in part on the observedfluorescence recovery after photobleaching (FRAP) alongvimentin IF networks in cultured cells [29]. Further stu-dies will be necessary to determine whether IF subunits oroligomers can exchange along individual filaments in cellsand tissues, or if exchange occurs at filament ends, orwhether both scenarios occur. Mechanisms that governin vivo assembly and remodeling are also beginning toemerge. It has been suggested that phosphorylation ofthe vimentin end domains might regulate IF assembly
ar organelles and cell-surface junctional structures are highlighted. IF link through
re they associate with mitochondria, Golgi membranes [e.g. formiminotransferase
-3 (AP-3)] [1,5,89]. IF might also position microtubule organizing centers (MTOCs),
[90], thus influencing MT organization and proper trafficking and distribution of
31
Box 1. Intermediate filament mechanical properties
MTs, MFs and IFs all contribute to cell structure, however IFs exhibit
properties that make them uniquely suited for maintaining cell
integrity in the face of mechanical and environmental stresses. IFs
are more flexible than MTs or MFs. This flexibility might be because
of the short persistence length of IFs compared with actin or MTs;
that is, IFs curve along their length more than MTs and MFs [12].
Compared with MTs and MFs, which rupture with small amounts of
strain, neurofilaments, desmin and some keratins have been
observed to extend to two to three times their original length,
becoming thinner and increasing in stiffness in response to the
strain placed on the filament. This extensibility might be due in part
to intrafilament sliding, a property unique to IFs because of the
longitudinal annealing of filament subunits [1,11,12,92,93]. Remark-
ably, unlike the other networks, IFs can recover from large amounts
of strain, thus the intrafilament sliding and resultant thinning is to
some extent reversible. Interactions among the IF, MT and MF
cytoskeletons can increase the resultant stiffness of the combined
networks. For example, the stiffness reported for vimentin and actin
mixtures is greater than that observed for either cytoskeleton alone
when subjected to the same force [94].
Review TRENDS in Cell Biology Vol.18 No.1
in vivo by altering the equilibrium constant of subunitexchange towards a higher off-rate [25,30]. Post-transla-tional modifications such as O-linked glycosylation andinteractions with IFAPs might also have an impact onthe dynamics of IF remodeling [2,25,31,32]. IFs thus havethe potential to be highly variable in structure and readilyremodeled in a dynamic, spatially regulated manner invivo.
The possible exchange of IF polypeptides into existingnetworks could help explain how remodeling of networksmight occur in vivo, but it doesn’t tell us how an IF networkforms de novo inside a cell. Recent studies using fluor-escent-tagged reporter proteins have identified non-fila-mentous ‘particles’ and small, filamentous forms of IFscalled ‘squiggles’ in cells, which can incorporate into thepolymerizing network [20,22,23,33,34]. Although thepossibility that these ectopically expressed probes couldinfluence normal IF dynamics should be kept in mind,these live-cell imaging studies have nevertheless beeninstructive in exposing potential mechanisms of assemblyinside a cell. The formation of putative IF precursors seemsto be a general property of IF because this has beenobserved for neurofilament proteins (NFs) [35–40], thetype III proteins peripherin [41,42] and vimentin[22,23,33,43] and some keratins [20,21,24,34,44–47].Particles seem to merge to form squiggles, however theprecise structure of the precursors observed in cells isunknown [23]. It is hypothesized that the particles andsquiggles contain aggregates of intermediates, such asULFs, and short filaments comprising polymerized ULFs,respectively [14,44,48,49].
Several ideas have been put forward to explain how andwhere IF precursors arise in cells. Precursors might formde novo from newly synthesized protein, or they mightassemble from a pre-existing soluble pool of protein, orboth. Support for a co-translationalmechanism comes fromrecent experiments carried out in rat PC12 cells, in whichtype III peripherin mRNA and its protein product wereimaged simultaneously and were shown to assemble intopresumptive IF precursor particles. After particle for-mation, the associated mRNAs moved away in a MT-de-
32
pendent fashion, leaving the stationary particle behind[42]. Once separated from the mRNA, these particles arealso capable of rapid MT-dependent translocation. Evi-dence for post-translational precursor formation in fila-ment turnover also exists. One example in support of apre-existing pool of IF subunits comes from the observationthat type II keratins are produced earlier in embryogenesisthan their type I partners, and are stored in aggregatesuntil heterodimerization takes place in the correcttemporal sequence [50]. Thus it is possible that both co-and post-translational mechanisms for precursor assemblyare used in cells, providing multiple modes for regulatingIF network formation.
Although various subcellular locations throughout thecell have been proposed as IF organizing centers, the cellperiphery has been identified as a ‘hot spot’ for initiation ofIF assembly. Fluorescent-labeled keratin probes have beenshown to incorporate into small particles in the actin-richperiphery of epithelial cells and move towards the cellcenter, converting into rodlets and eventually integratinginto the existing IF network [45]. The idea that the IFnetwork can be replenished primarily by addition of pre-cursors at the network periphery was supported by FRAPanalysis. Together, these observations suggest anotherway in which existing networks might be remodeled incells. Recently, both vimentin and keratin precursors havebeen shown to form in close proximity to focal complexes atsites of close apposition to the cell substrate [43,47,51] andthey seem to move along associated actin fibers [47].Intriguingly, talin-deficient focal adhesions do not supportcortical keratin precursor formation and dynamics.Although it seems possible that indirect effects owing toalterations in focal contact-mediated adhesion or signalingmight contribute to impaired IF behavior, collectively thedata support an important role for the cell periphery in IFdynamics [47]. The ability to initiate precursor formationfrom multiple cellular locations might be important forcustomizing the IF network with high spatial resolution toallow structural and signaling functions to be targeted tospecific cell compartments.
IF precursors: on the moveUnlikeMTs andMFs, IFs do not seem to serve as tracks formovement of membrane vesicles and other cellular traffic.IF particles are themselves cargo that can be movedaround the cell by various motor proteins. The direction,rate ofmovement and pause frequency vary as a function ofIF type, cell type and size of IF precursor (Figure 3). TypeIV NFs and type III vimentin and peripherin have severaldynamic behaviors in common. They have each beenobserved to move �60% of the time in the anterogradeand �40% of the time in the retrograde direction[35,36,40,41]. MT motors in the kinesin family have beenimplicated in anterograde translocation of vimentin[22,33,48,52,53] and NF [37,54–58] whereas dynein anddynactin are part of a retrograde complex, which inneurons might be mediated by an interaction betweenthe neurofilament medium chain (NF-M) and the dyneinintermediate chain [40,41,57–60].
Vimentin and peripherin exhibit particle speeds ran-ging between�0.3 and 0.4 mm/s consistent with trafficking
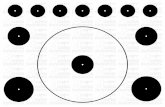
Figure 3. Schematic representation of the interaction between IF precursors with microtubule- and actin-associated motor complexes. Type III and type IV IF precursors are
observed in two forms, non-filamentous particles and small filament squiggles. Particles are formed cotranslationally in the cytoplasm and the mRNP moves away from the
precursor, which then can associate with the network at the site of synthesis or can be moved to distant incorporation sites via a molecular motor [42]. mRNPs appear to
travel along MTs, but the motor interaction is not yet known. Kinesin presumably directs anterograde movements of IF particles and squiggles, whereas dynein and
dynactin mediate retrograde mobility [48]. No motors have yet been implicated in keratin particle and squiggle translocation, however the precursors seem to use MT and
actin. Actin also seems to have a role in NF translocation as myosin Va binds to NF-L, and such associations with MF might regulate the frequency of NF precursor
movements [68]. This schematic highlights interactions between IF and motor complexes and is not drawn to scale.
Review TRENDS in Cell Biology Vol.18 No.1
by conventional MT motors, whereas squiggle movementcan be much slower, between 0.06 and 0.3 mm/s[22,23,33,41,61]. Particles and squiggles of all IF typescan pause during translocation, sometimes up to �80% ofthe time [21,35,36,41]. For example, NF precursorswere once believed to be translocated by a mechanismdistinct from the faster, MT-based transport observed forother proteins, but live cell imaging has recently shownthat they actually move in bursts of speed at >1 mm/sinterspersed with long, frequent pauses [35–37,58,62,63].The underlying basis of the observed differences in speedand pause times between IF types is not well understood.It has been speculated that transient associations withMT- or IF-associated proteins might put a drag on theparticles, or that a change in the phosphorylation state ofIFs regulates their translocation properties [25,58] per-haps by affecting interactions with the translocationmachinery. In support of this hypothesis, NF C-terminalphosphorylation can modulate precursor dynamics[54,57,58,64–66].
Keratin precursors have been reported to move morefrequently in the retrograde direction than type III and IVIFs [20–23,33,47,61]. In addition, keratin precursorkinetics can be divided into two classes, one with slowkinetics (0.001–0.01 mm/s) requiring actin and one withmore rapid kinetics (>0.01 mm/s) requiring MTs [46]. Insupport of a physiological role for actin in IF dynamics,keratins were shown to move in concert with F-actin inXenopus extracts. Furthermore, IF polymerization in theseextracts, as well as in activated oocytes, required F-actin,perhaps for proper IF precursor alignment and exchangeinto the polymerizing filament [67]. A role for a specificmyosin motor has been demonstrated in mice harboring amyosin Va mutation, as the animals exhibit a higherdensity of NF in the cytoplasm associated with a modestincrease in NF protein levels [68]. Likewise, myosin Va isaberrantly distributed in mice deficient in NF-L, a bindingpartner and potential cargo for thismyosin, and it has beenproposed that an NF–myosin Va interaction regulates thefrequency of NF precursor movements [68].
33
Review TRENDS in Cell Biology Vol.18 No.1
IFs and IFAPs: partners in regulation of cytoskeletalnetworksIFAPs are emerging as important regulators of IF networkremodeling and function, particularly members of theplakin family. Members of this family have been describedas structural linkers between IF and cell–cell or cell–sub-strate adhesions and as crosslinkers with other cyto-skeletal elements [69]. The linkages are facilitated bythe modular structure of the family members, each ofwhich contains a ‘mix-and-match’ collection of differentbuilding blocks, including IF-binding, actin-binding(ABD), coiled-coil rod, spectrin repeat-containing andMT-binding domains, as well as domains that target cer-tain members to junctions such as desmosomes or hemi-desmosomes.
One plakin, known as plectin, is a large family membercontaining domains that enable it to associate with andcrosslink all three cytoskeletal systems. These domainsinclude an N-terminal ABD, built from two N-terminalcalponin homology (CH) domains and a C-terminal IF-binding domain. Highlighting its importance in organizingIF, plectin-deficient cells lose proper orthogonal IF cross-linking making them more susceptible to stress-induceddisruption of the IF cytoskeleton and accompanyingchanges in cell signaling and cell migration [70].
Plectin might also regulate IF polymerization anddynamics. In addition to its C-terminus, a second IF-bind-ing site was recently discovered within the plectin N-terminus in the first CH domain. However, this site cannotbind filamentous vimentin, which prompted investigatorsto suggest that itmight instead regulate the dynamics of IFpolymerization [71]. Such a function might contribute tothe observed nucleation of vimentin at matrix adhesions[43,51]. Other CH domain-containing proteins such asfimbrin, which colocalizes with vimentin in podosomes,and calponin, which interacts with desmin, are thoughtto bind tetrameric forms of IF with a higher affinity thanpolymerized filaments. These observations highlight apotential conserved function for CH domain-containingproteins in regulating IF assembly [4].
Recent additions to the plakin family – periplakin andepiplakin – might also contribute to IF organization andassembly state. Epiplakin deficiency resulted in destabi-lization of simple epithelial networks in cultured cells andacceleration of keratinocyte migration in mice [72,73].Interestingly, siRNA-mediated knock-down of the familymember periplakin inhibited proper keratin organizationand also inhibited wound closure in vitro [32]. Althoughfurther work will be needed to understand how these andother associated proteins control IF assembly at a molecu-lar level, these observations collectively support a generalrole for IFAPs in regulating IF networks.
Similarly, plakin protein dynamics might be regulatedby their interactions with IFs, as is the case with desmo-plakin (DP) during its assembly into desmosomes [74]. Thisplakin protein has been shown to be required for anchoringIF to the desmosomal cadherin complex at the plasmamembrane of epithelial cells [75,76]. However, its associ-ationwith IF has also been shown to regulate the trajectoryand efficiency of DP precursor particle movement from thecytoplasm into nascent desmosomes following cell–cell
34
contact [74,77]. The precise molecular mechanism oftranslocation is not known, but it has been shown to beactin-dependent. Based on previous data raising thepossibility that DP can co-assemble with IF in vivo,possibly by forming co-oligomers, it seems possible thatDP could co-assemble with and piggy-back on IF particlesor squiggles during the assembly process and be translo-cated by some mechanism requiring actin. Alternatively,DP particles enmeshed in the IF filament network mightbe brought into proximity with the cell borders by thecontraction or remodeling of cortical actomyosin, whichis coupled to the assembly of a related cell–cell junction,the adherens junction [78,79].
IFs and disease: relationship with dynamics andassemblyDemonstration that IFs provide mechanical integrity totissues first came from pioneering studies showing thatmutations in K5 and 14 led to epidermal fragility in miceand epidermolysis bullosa simplex (EBS) in humans [6].Similar mutations (although distributed differently alongthe length of the polypeptide chain) have since then beenfound in other IF family members, leading to identificationof a plethora of disease phenotypes, at least some of whichare due to fragility because of loss of IF network function(Table 1) [10] (Human Intermediate Filament MutationDatabase, http://www.interfil.org).
Early in vitro studies supported the idea that keratinmutations, which were frequently seen to be clustered inthe highly conserved rod end domains, led to defects inpolymer assembly (reviewed in [6]). Supporting the ideathatmutations in type III IFs also affect polymer assembly,desmin mutations that result in severe myopathies wereshown to alter filament assembly at different stages of ULFformation [80], mutations in glial fibrillary acidic protein(GFAP) impair assembly in vitro and in cells lacking otherIF [81], and NF-L mutations linked to Charcot-Marie-Tooth disease result in defects in assembly and networkformation [82]. At least some keratin mutant proteins actin a dominant negative way, increasing the soluble pool ofsubunits and leading to defects in the IF network and theformation of cytoplasmic aggregates (reviewed in [2,10]).Not all IF mutations behave in this way. For example,GFAP mutants that cause Alexander disease, which ischaracterized by cytoplasmic inclusions in astrocytescalled Rosenthal fibers, are resistant to detergent extrac-tion [81,83].
Recently there has been discussion regarding whetherimpaired assembly and/or network formation is a hallmarkof all IF mutations, particularly in the presence of a wild-typepartner.Forexample, the simpleepithelialkeratinK18mutation R89C leads to network disruption in vivo, render-ing hepatocytes fragile and predisposing these cells to apop-tosis. By contrast, the presence of a G61C mutation in thepartner protein K8 does not result in detectable changes inIF networks or hepatocyte fragility, yet still predisposesthese cells to apoptosis [84]. Similarly, although some dis-ease-causing neurofilament mutations result in assemblydefects, aG336Svariant in the roddomainof thehumanNF-M protein present in a patient with Parkinson’s disease didnot [85]. Furthermore, desmin mutants co-polymerize with
Review TRENDS in Cell Biology Vol.18 No.1
wild-type protein in physiologically relevant concentrations[86] and keratin mutants have also been shown to formfilaments in vitro without detectable defects [15]. Eventhough mutant IF and IF networks might be perceived assuperficially ‘normal’ in vitro or in cells, it seems possiblethat structural defects might be unmasked by physicalstress applied in vitro or experienced in vivo.
These observations underscore the emerging realizationthat mutations can contribute to disease in ways that gobeyond formation of the polymer. For instance, it wasshown that a K14 ‘hotspot’ mutation impairs the abilityof mutant filaments to bundle and reduces resilience of thenetwork [87]. This defect in bundling could contribute tothe observed collapse of the filament system seen in vivo.Mutation of binding sites for other network constituentscould also affect IF network formation. For example, anI451Mmutation within the type III desmin tail (associatedwith cardiomyopathy) that exhibited only partial impair-ment of IF network formationwas recently shown to lead toa lack of binding to the junctional plaque protein desmo-plakin in vitro [88]. Mutations in the KI tail lead to severepalmoplantar hyperkeratosis resulting from uncouplingwith the cornified envelope protein loricrin (reviewed in[6]). Interestingly, a similar ichthyosis results from loricrinmutations and cardiomyopathies can arise as a result ofmutations in the desmoplakin-binding domain of desmin.
These observations have led to the concept of ‘pheno-typic convergence’ in which similar phenotypes arise frommutations in an IF family member or its associatedproteins [2]. Other examples of this sort of convergenceare: (i) mutations in plectin lead to a type of EBS withmuscular dystrophy; (ii) mutations in desmin-associatedaB-crystallin results in cardiomyopathy; and (iii) type 2Charcot-Marie-Tooth can be caused by mutations in NF-L,lamin A/C, or the IF-associated heat shock protein hsp27[2] (Human Intermediate Filament Mutation Database;http://www.interfil.org). Finally, the NF-L-binding proteinNUDEL might emerge as another example of this concept.This protein destabilizes NF-L in neuronal cells resultingin NF defects and morphological changes similar to thoseobserved in neurodegeneration [19].
Just as live-cell imaging has revealed dynamic beha-viors of wild-type IFs in cells, it has also suggestedadditional ways in which disease mutations might impairIF function. Imaging of a yellow fluorescent protein-taggedK14 R125C mutant found in patients with EBS showedthat this mutant participates in the formation of highlydynamic, short-lived aggregates containing both mutantand wild-type protein. These seemmorphologically similarto aggregates observed in vivo. Aggregates in the peripheryof the cell cytoplasm flow inward via an actin-dependentmechanism, are transient in nature, and are in equilibriumwith a soluble pool of increased magnitude [34,45]. Theaggregates are mostly segregated from the centrallylocated filamentous network (thought to contain a higherproportion of wild-type protein that the aggregates) thatco-exists in these cells and that seems to be less dynamicand more susceptible to environmental stress than controlnetworks. It was suggested that the zone in which aggre-gates form might be equivalent to the zone of lysis in thebasal layer of epidermis.
As we continue to probe the structural basis for diseasephenotypes it is also becoming clear that the complexity ofgenotype–phenotype relationships is increasing with theemergence of non-mechanical functions for IFs [2,25,63].In the future it will be interesting to determine the fre-quency with which the various functions of IFs are corre-lated with their assembly state, and whether mutationsthat shift the ratio of different oligomeric forms of IF in acell preferentially affect mechanical versus non-mechan-ical functions.
Conclusion and future challengesThe studies reviewed here provide some hints as to therichness and diversity of IF dynamics but we have far to gobefore these mechanisms are completely unraveled, evenfor a single type of IF. Advances in fluorescence microscopyhave enhanced the resolution of visualization such thatsingle molecules can now be tracked in vivo, enablingresearchers to put the current IF assembly model to thetest in physiological situations. How IFAPs contribute toIF initiation and polymerization, as well as the formationand mechanical properties of integrated cytoskeletal net-works involving actin and MTs, are questions that willincreasingly guide experiments in the coming years.Although our understanding of MT and MF dynamicshas been facilitated by high-resolution electron microscopyand X-ray crystallographic analysis, the 3D structure ofIFs remains elusive [1]. However, by applying emergingtechniques such as cryo-electron tomography and atomicforce microscopy (AFM), along with the continued use of IFfragments for X-ray crystallographic analysis, it isexpected that the structure and nanomechanics of IFpolypeptides will continue to be unraveled. In the future,production of co-crystals of IF fragments and their associ-ated proteins, coupled with analytical biochemistry, willenable us to determine how these interactions affect thepolymerization dynamics of IF and lead to the assembly ofnovel fibrous structures such as junctional plaques.
AcknowledgementsThe authors are grateful to colleagues in the field for their contributionsto the work discussed here and apologize to those whose work we wereunable to cite owing to lack of space. The authors also thank P. Coulombeand B. Omary for helpful discussions. K.J.G. is supported by grantsR01AR43380, R01AR41836, R01CA122151, project #4 of P01DE12328and the J.L. Mayberry Endowment.
References1 Herrmann, H. et al. (2007) Intermediate filaments: from cell
architecture to nanomechanics. Nat. Rev. Mol. Cell Biol. 8, 562–5732 Kim, S. and Coulombe, P.A. (2007) Intermediate filament scaffolds
fulfill mechanical, organizational, and signaling functions in thecytoplasm. Genes Dev. 21, 1581–1597
3 Parry, D.A. et al. (2007) Towards a molecular description ofintermediate filament structure and assembly. Exp. Cell Res. 313,2204–2216
4 Green, K.J. et al. (2005) Intermediate filament associated proteins.Adv. Protein Chem. 70, 143–202
5 Toivola, D.M. et al. (2005) Cellular integrity plus: organelle-related andprotein-targeting functions of intermediate filaments. Trends Cell Biol.15, 608–617
6 Magin, T.M. et al. (2004) Emerging functions: diseases and animalmodels reshape our view of the cytoskeleton.Exp. Cell Res. 301, 91–102
7 Omary, M.B. et al. (2004) Intermediate filament proteins and theirassociated diseases. N. Engl. J. Med. 351, 2087–2100
35
Review TRENDS in Cell Biology Vol.18 No.1
8 Mattout, A. et al. (2006) Nuclear lamins, diseases and aging. Curr.Opin. Cell Biol. 18, 335–341
9 Worman, H.J. and Bonne, G. (2007) Laminopathies: a wide spectrum ofhuman diseases. Exp. Cell Res. 313, 2121–2133
10 Magin, T.M. et al. (2007) Structural and regulatory functions ofkeratins. Exp. Cell Res. 313, 2021–2032
11 Kreplak, L. and Fudge, D. (2007) Biomechanical properties ofintermediate filaments: from tissues to single filaments and back.Bioessays 29, 26–35
12 Wagner, O.I. et al. (2007) Softness, strength and self-repair inintermediate filament networks. Exp. Cell Res. 313, 2228–2235
13 Sokolova, A.V. et al. (2006) Monitoring intermediate filament assemblyby small-angle x-ray scattering reveals the molecular architecture ofassembly intermediates.Proc.Natl.Acad.Sci.U.S.A.103, 16206–16211
14 Mucke, N. et al. (2004) Molecular and biophysical characterization ofassembly-starter units of human vimentin. J. Mol. Biol. 340, 97–114
15 Herrmann, H. et al. (2002) Characterization of early assemblyintermediates of recombinant human keratins. J. Struct. Biol. 137,82–96
16 Herrmann, H. and Aebi, U. (2004) Intermediate filaments: molecularstructure, assembly mechanism, and integration into functionallydistinct intracellular scaffolds. Annu. Rev. Biochem. 73, 749–789
17 Parry, D.A. (2005) Microdissection of the sequence and structure ofintermediate filament chains. Adv. Protein Chem. 70, 113–142
18 Gu, L.H. and Coulombe, P.A. (2005) Defining the properties of thenonhelical tail domain in type II keratin 5: insight from a bullousdisease-causing mutation. Mol. Biol. Cell 16, 1427–143818
19 Nguyen, M.D. et al. (2004) A NUDEL-dependent mechanism ofneurofilament assembly regulates the integrity of CNS neurons.Nat. Cell Biol. 6, 595–608
20 Windoffer, R. and Leube, R.E. (1999) Detection of cytokeratin dynamicsby time-lapse fluorescence microscopy in living cells. J. Cell Sci. 112,4521–4534
21 Yoon, K.H. et al. (2001) Insights into the dynamic properties of keratinintermediate filaments in living epithelial cells. J. Cell Biol. 153, 503–516
22 Prahlad, V. et al. (1998) Rapid movements of vimentin on microtubuletracks: kinesin-dependent assembly of intermediate filamentnetworks. J. Cell Biol. 143, 159–170
23 Yoon, M. et al. (1998) Motile properties of vimentin intermediatefilament networks in living cells. J. Cell Biol. 143, 147–157
24 Windoffer, R. and Leube, R.E. (2001) De novo formation of cytokeratinfilament networks originates from the cell cortex in A-431 cells. CellMotil. Cytoskeleton 50, 33–44
25 Omary, M.B. et al. (2006) ‘Heads and tails’ of intermediate filamentphosphorylation: multiple sites and functional insights. TrendsBiochem. Sci. 31, 383–394
26 Sahlgren, C.M. et al. (2001) Mitotic reorganization of the intermediatefilament protein nestin involves phosphorylation by cdc2 kinase.J. Biol. Chem. 276, 16456–16463
27 Chou, Y.H. et al. (2007) The motility and dynamic properties ofintermediate filaments and their constituent proteins. Exp. Cell Res.313, 2236–2243
28 Ridge, K.M. et al. (2005) Keratin 8 phosphorylation by protein kinase Cdelta regulates shear stress-mediated disassembly of keratinintermediate filaments in alveolar epithelial cells. J. Biol. Chem.280, 30400–30405
29 Vikstrom, K.L. et al. (1992) Steady state dynamics of intermediatefilament networks. J. Cell Biol. 118, 121–129
30 Eriksson, J.E. et al. (2004) Specific in vivo phosphorylation sitesdetermine the assembly dynamics of vimentin intermediatefilaments. J. Cell Sci. 117, 919–932
31 Izawa, I. and Inagaki, M. (2006) Regulatory mechanisms and functionsof intermediate filaments: A study using site- and phosphorylationstate-specific antibodies. Cancer Sci. 97, 167–174
32 Long, H.A. et al. (2006) Periplakin-dependent re-organisation ofkeratin cytoskeleton and loss of collective migration in keratin-8-downregulated epithelial sheets. J. Cell Sci. 119, 5147–5159
33 Martys, J.L. et al. (1999) Intermediate filaments in motion:observations of intermediate filaments in cells using greenfluorescent protein-vimentin. Mol. Biol. Cell 10, 1289–1295
34 Werner, N.S. et al. (2004) Epidermolysis bullosa simplex-typemutations alter the dynamics of the keratin cytoskeleton and reveal
36
a contribution of actin to the transport of keratin subunits. Mol. Biol.Cell 15, 990–1002
35 Roy, S. et al. (2000) Neurofilaments are transported rapidlybut intermittently in axons: implications for slow axonal transport.J. Neurosci. 20, 6849–6861
36 Wang, L. et al. (2000) Rapid movement of axonal neurofilamentsinterrupted by prolonged pauses. Nat. Cell Biol. 2, 137–141
37 Yabe, J.T. et al. (1999) Kinesin-mediated transport of neurofilamentprotein oligomers in growing axons. J. Cell Sci. 112, 3799–3814
38 Yan, Y. and Brown, A. (2005) Neurofilament polymer transport inaxons. J. Neurosci. 25, 7014–7021
39 Wang, L. and Brown, A. (2001) Rapid intermittent movement of axonalneurofilaments observed by fluorescence photobleaching. Mol. Biol.Cell 12, 3257–3267
40 Shah, J.V. et al. (2000) Bidirectional translocation of neurofilamentsalong microtubules mediated in part by dynein/dynactin. Mol. Biol.Cell 11, 3495–3508
41 Helfand, B.T. et al. (2003) Rapid transport of neural intermediatefilament protein. J. Cell Sci. 116, 2345–2359
42 Chang, L. et al. (2006) Assembling an intermediate filament networkby dynamic cotranslation. J. Cell Biol. 172, 747–758
43 Tsuruta, D. and Jones, J.C. (2003) The vimentin cytoskeleton regulatesfocal contact size and adhesion of endothelial cells subjected to shearstress. J. Cell Sci. 116, 4977–4984
44 Liovic, M. et al. (2003) Observation of keratin particles showing fastbidirectional movement colocalized with microtubules. J. Cell Sci. 116,1417–1427
45 Windoffer, R. et al. (2004) Identification of novel principles of keratinfilament network turnover in living cells.Mol. Biol. Cell 15, 2436–2448
46 Woll, S. et al. (2005) Dissection of keratin dynamics: differentcontributions of the actin and microtubule systems. Eur. J. CellBiol. 84, 311–328
47 Windoffer, R. et al. (2006) Focal adhesions are hotspots for keratinfilament precursor formation. J. Cell Biol. 173, 341–348
48 Chang, L. and Goldman, R.D. (2004) Intermediate filaments mediatecytoskeletal crosstalk. Nat. Rev. Mol. Cell Biol. 5, 601–613
49 Kirmse, R. et al. (2007) A quantitative kinetic model for the in vitroassembly of intermediate filaments from tetrameric vimentin. J. Biol.Chem. 282, 18563–18572
50 Lu, H. et al. (2005) Type II keratins precede type I keratins during earlyembryonic development. Eur. J. Cell Biol. 84, 709–718
51 Gonzales, M. et al. (2001) Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol. Biol. Cell 12,85–100
52 Kreitzer, G. et al. (1999) Detyrosination of tubulin regulates theinteraction of intermediate filaments with microtubules in vivo via akinesin-dependent mechanism. Mol. Biol. Cell 10, 1105–1118
53 Liao, G. and Gundersen, G.G. (1998) Kinesin is a candidate for cross-bridgingmicrotubules and intermediate filaments. Selective binding ofkinesin to detyrosinated tubulin and vimentin. J. Biol. Chem. 273,9797–9803
54 Yabe, J.T. et al. (2000) Phospho-dependent association ofneurofilament proteins with kinesin in situ. Cell Motil. Cytoskeleton45, 249–262
55 Theiss, C. et al. (2005) Impairment of anterograde and retrogradeneurofilament transport after anti-kinesin and anti-dynein antibodymicroinjection in chicken dorsal root ganglia. Eur. J. Cell Biol. 84,29–43
56 Xia, C.H. et al. (2003) Abnormal neurofilament transport caused bytargeted disruption of neuronal kinesin heavy chain KIF5A. J. CellBiol. 161, 55–66
57 Motil, J. et al. (2006) Dynein mediates retrograde neurofilamenttransport within axons and anterograde delivery of NFs fromperikarya into axons: regulation by multiple phosphorylation events.Cell Motil. Cytoskeleton 63, 266–286
58 Sihag, R.K. et al. (2007) Role of phosphorylation on the structuraldynamics and function of types III and IV intermediate filaments.Exp.Cell Res. 313, 2098–2109
59 Helfand, B.T. et al. (2002) A requirement for cytoplasmic dynein anddynactin in intermediate filament network assembly and organization.J. Cell Biol. 157, 795–806
60 Wagner, O.I. et al. (2004) The interaction of neurofilaments with themicrotubule motor cytoplasmic dynein. Mol. Biol. Cell 15, 5092–5100
Review TRENDS in Cell Biology Vol.18 No.1
61 Helfand, B.T. et al. (2004) Intermediate filaments are dynamic andmotile elements of cellular architecture. J. Cell Sci. 117, 133–141
62 Shea, T.B. (2000) Microtubule motors, phosphorylation and axonaltransport of neurofilaments. J. Neurocytol. 29, 873–887
63 Brown, A. (2000) Slow axonal transport: stop and go traffic in the axon.Nat. Rev. Mol. Cell Biol. 1, 153–156
64 Jung, C. et al. (2000) Hypophosphorylated neurofilament subunitsundergo axonal transport more rapidly than more extensivelyphosphorylated subunits in situ. Cell Motil. Cytoskeleton 47, 120–129
65 Jung, C. et al. (2000) C-terminal phosphorylation of the high molecularweight neurofilament subunit correlates with decreased neurofilamentaxonal transport velocity. Brain Res. 856, 12–19
66 Ackerley, S. et al. (2003) Neurofilament heavy chain side armphosphorylation regulates axonal transport of neurofilaments.J. Cell Biol. 161, 489–495
67 Weber, K.L. and Bement, W.M. (2002) F-actin serves as a template forcytokeratin organization in cell free extracts. J. Cell Sci. 115, 1373–1382
68 Rao,M.V. et al. (2002)Myosin Va binding to neurofilaments is essentialfor correct myosin Va distribution and transport and neurofilamentdensity. J. Cell Biol 159, 279–289
69 Sonnenberg, A. and Liem, R.K. (2007) Plakins in development anddisease. Exp. Cell Res. 313, 2189–2203
70 Osmanagic-Myers, S. et al. (2006) Plectin-controlled keratincytoarchitecture affects MAP kinases involved in cellular stressresponse and migration. J. Cell Biol. 174, 557–568
71 Sevcik, J. et al. (2004) Actin-binding domain of mouse plectin. Crystalstructure and binding to vimentin. Eur. J. Biochem. 271, 1873–1884
72 Jang, S.I. et al. (2005) Characterization of human epiplakin: RNAi-mediated epiplakin depletion leads to the disruption of keratin andvimentin IF networks. J. Cell Sci. 118, 781–793
73 Goto, M. et al. (2006) Elimination of epiplakin by gene targeting resultsin acceleration of keratinocyte migration in mice. Mol. Cell. Biol. 26,548–558
74 Godsel, L.M. et al. (2005) Desmoplakin assembly dynamics in fourdimensions: multiple phases differentially regulated by intermediatefilaments and actin. J. Cell Biol. 171, 1045–1059
75 Vasioukhin, V. et al. (2001) Desmoplakin is essential in epidermalsheet formation. Nat. Cell Biol. 3, 1076–1085
76 Huen, A.C. et al. (2002) Intermediate filament-membrane attachmentsfunction synergistically with actin-dependent contacts to regulateintercellular adhesive strength. J. Cell Biol. 159, 1005–1017
77 Loranger, A. et al. (2006) Keratin 8 modulation of desmoplakindeposition at desmosomes in hepatocytes. Exp. Cell Res. 312, 4108–4119
78 Zhang, J. et al. (2005) Actin at cell-cell junctions is composed of twodynamic and functional populations. J. Cell Sci. 118, 5549–5562
79 Yamada, S. and Nelson, W.J. (2007) Localized zones of Rho and Racactivities drive initiation and expansion of epithelial cell cell adhesion.J. Cell Biol. 178, 517–527
80 Bar, H. et al. (2005) Severe muscle disease-causing desmin mutationsinterfere with in vitro filament assembly at distinct stages. Proc. Natl.Acad. Sci. U. S. A. 102, 15099–15104
81 Hsiao, V.C. et al. (2005) Alexander-disease mutation of GFAP causesfilament disorganization and decreased solubility of GFAP. J. Cell Sci.118, 2057–2065
82 Perez-Olle, R. et al. (2004) Phenotypic analysis of neurofilament lightgene mutations linked to Charcot-Marie-Tooth disease in cell culturemodels. Hum. Mol. Genet. 13, 2207–2220
83 Quinlan, R.A. et al. (2007) GFAP and its role in Alexander disease.Exp.Cell Res. 313, 2077–2087
84 Ku, N.O. and Omary, M.B. (2006) A disease- and phosphorylation-related nonmechanical function for keratin 8. J. Cell Biol. 174, 115–125
85 Perez-Olle, R. et al. (2004) The G336S variant in the humanneurofilament-M gene does not affect its assembly or distribution:importance of the functional analysis of neurofilament variants.J. Neuropathol. Exp. Neurol. 63, 759–774
86 Bar, H. et al. (2007) Assembly defects of desmin disease mutantscarrying deletions in the alpha-helical rod domain are rescued bywild type protein. J. Struct. Biol. 158, 107–115
87 Ma, L. et al. (2001) A ‘hot-spot’ mutation alters the mechanicalproperties of keratin filament networks. Nat. Cell Biol. 3, 503–506
88 Lapouge, K. et al. (2006) New insights into the molecular basis ofdesmoplakin- and desmin-related cardiomyopathies. J. Cell Sci. 119,4974–4985
89 Styers, M.L. et al. (2005) Intermediate filaments and vesicularmembrane traffic: the odd couple’s first dance? Traffic 6, 359–365
90 Oriolo, A.S. et al. (2007) GCP6 binds to intermediate filaments: a novelfunction of keratins in the organization of microtubules in epithelialcells. Mol. Biol. Cell 18, 781–794
91 Wu, K.C. et al. (2000) Coiled-coil trigger motifs in the 1B and 2B roddomain segments are required for the stability of keratin intermediatefilaments. Mol. Biol. Cell 11, 3539–3558
92 Kreplak, L. et al. (2005) Exploring the mechanical behavior of singleintermediate filaments. J. Mol. Biol. 354, 569–577
93 Storm, C. et al. (2005) Nonlinear elasticity in biological gels. Nature435, 191–194
94 Esue, O. et al. (2006) A direct interaction between actin and vimentinfilaments mediated by the tail domain of vimentin. J. Biol. Chem. 281,30393–30399
37