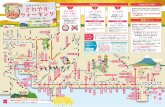
C23 - Yamato・ C1 やまと公園・ 深見北 コミュニティー センター 大和小学校 市立病院 鶴間駅 大和駅→鶴間駅 鶴間駅→大和駅 C25 鶴間コミュニティーセンター
ユナイテッドクリニック新橋駅前院【医療法人社団淳康会・ …The Hong Kong...
Transcript of ユナイテッドクリニック新橋駅前院【医療法人社団淳康会・ …The Hong Kong...





国STE汽 :墜__塁」は堅輯_
I])atc :2018-06-01
No.:HC18051277
Applicant(Code:01311126): Succccd Holdings Ltd
蚕湾沙咀道 1…9琥永南貨倉大度 18棲
Description of Sample(s)
Page 1 of 1
One subnlitted sainple said to be(〕difast 120img 2x21 capsules.
Batchノ Lot Nb.:7E190B ‐
Exp Datc:06/2020COuntry Of Ori」 n i PhilippinCS
Da“ Sal■ple(s)Received : 2018-05‐ 25
Date Tested 2018-05-30 to 2018‐05‐31
Investigation Requested : Orlistat
Method(s)Used
Test Result(s)
High Performance Liquid Chromatography
TeSt ltem(S) Result
Orlist江 121.8 mg/capsule
Note : Average filled weight per capsule :0.23739
KWOIK Nga Y薇 1,Benlice
Authorized Signatory
Cheinical and Food Depaltinent
For and on bchalf of
The IIong Kong Standards and Tcsing Centrc Ltd.
*****End of TestiReport*****
The Hong Kong Standards and Testing Centre Limited
10 DaiWang $reet, Tai Po lndustrial Estate, Tai Po, N.T., Hong Kong
Tel: +852 2666 1888 Fax: +852 2664 4353 E-mail [email protected] Website: www.stc.group
This report shall not be reproduced unless with prior written approval {rom The Hong Kong Standards and Testing Centre Limited.
For Conditions o{ lssuance of this test report, please refer to the overleai or Website
Sample(s) Received Condition: In intact original package underambient temperature

轟一覇
欝欝熙
STC Test R222rt
I:)ate :2015-09-08
No.:HC373862
Applicant(Code:SUH017): Succeed Holdings Ltd
杢湾沙咀道 1‐9琥永南貨倉大度 18棲
Description of Sample(s) One submitted sample said to be Tadacip.BatchlLotNo.: BT404lPack Size : 4 tablets/boxExp Date: Jan 16Country of Origin : India
Sample(s) Received Condition:
Page 1 of I
In intact original package underambient temperature
Date Sample(o Received : 2015‐ 08‐28
Date Tested 2015¨ 09日 04
Investigation Requested : Tadalafil
Method(s) Used
Test Result(s)
High Performance Liquid Chromatography
旦[笠旦‐1_ltem(S) Result
Tad」 a■ 1 20.3 mg/tあ let
Note: Average weight= 0.3457 g/tabler
:,
TAM Leong Hang, AnthonyAuthorized Signatory
Chemical and Food DepartmenrFor and on behalf of
The Hong Kong Standards and Testing Centre Ltd.
***** End of Test Report 't'r"**
The Hon9 KOng Standards and Testing Centre L『 nled
10 DaiVVang(Street,TaI Po lndus[ial EState,Tal Po,NT,|→ ong Kong
■el:+852 2666 1888 Fax:● 85226644353 E‐maili hkstc@hkstc org 〃ヽebsite:www stC‐ 9「Oup Org
ThiS repOrt Sha‖ nOt be reprOduCed unleSS Wth priOr'Written approvalfrom The Hong Kong(■ andards and■ esting Centre Limmed
For Condnions oflssuance ofthis test re100rt,please referto the oveneaf or VVlebsite


_ _||■ ‐ ‐|■ _‐ . .
Date 1 2015‐ 03-16
No.:HC361308Page 1 of 1
Applicant {Code: SUH017) : Succeed Holdings LtdWorkshop I. J, K, L Superluck Ind Ctr Plrase II57 Sha Tsui RdTsuen Wan NT HK
Description af Sam ple{s) One submitted sample said to be Sildenafil l00rng Filrn-coatedTablets.Elatch / Lot No.: 3A279718Pack Size : 4 tablets/boxCountry of Origin : France
Sarnple{s) Received Condition: In ir:tact original package underambierrt fenrperature
Datr Sanr;rle(s) Rcceived : 201 ,5-il3- 12
Dnte Testerl : 2015-03-16
番llvesti=atio甕 1貫lequeste(1 : Sil(lclla:11
則101:IttOd(S)USe(1 i l i gll Pe rfbnnan ce L i q r,r icl Cli rornatography,
Test f{,esnlt(si Test ltsm(s) Sildena』il 100nlg
Fil墨1…coated´Ilablets
Sil(lenail 99.0 nrg/tablet
Note: Average rveight: 0.6290 g/tahlet
KWOK Nga Yani BcrlllccAtlt1loHzed Signatory
Cheniical and Food DepaftnrentFor and on behalfof
The Hollg Iく o換 g Sta■ dal・ds and Tcsting Centre Ltd
****t< End Of TeSt Repoft 'trr'!**
The Hong Kong Standards and Testing Centre Limited
10 Dai Wang Street, Tai Po lnduslrial E$tats, Tai Po, N-T,, Hong Kong
Tel: +852 2666 1888 Fax: +852 2664 4353 Email: [email protected] Website: www.stc-group.org
This report shall not be reproduced unlees with prior written approval from The Hong Kong Siandard$ and Testing Centr8 Limit6d
For Conditions of lssuance ofthis test report, please refer to the overleaf and Websile.
/´`ヽ
/ ′ ヽ
ヽ _L/
____´´
КO●●

STC Test Ren2rt
I:)ate :2015-09… 08
No. ::HC373860
Applicant(Code: SUH017) : Succeed IIoldings]し td
杢湾沙咀道 1‐9琥永南貨倉大度 18棲
Description of Sample(s) : One submitted sample said to be Xenical 42 Cap.Batch I Lot No.: M20B7M3Pack Size : 42 capsulesExp Date : Jan 16Country of Origin : Italy
Page 1 of1
Sample(s) Received Condition: In intact original package underambient temperature
Date Sample(s) Received : 2015-08-28
Date Tested : 2015‐ 09‐02
Investigation Requested : Orlistat
Method(s)Used
Test Result(s)
: High Performance Liquid Chromatography
TeSt ltem(S) Result
Orlist誠 120 mg/capsule
Note : Average weight per capsule :0.24359
.` (メ `
■■ ´
~警、
:「
TAM lpong Hang, AnthonyAuthorized Signatory
Chemical and Food DepartmenrFor and on behalfof
The Hong Kong Standards and Testing Centre Ltd.
***?trr End Of TeSt RepO6 *,i***
The Hong Kong Standards and Tiesing Centre Limited
10 Dai VVang Street,■ai Po lndustnal Estate.Tal Po,N.T.Hong Kong
■ol:+852 2666 1888 Fax:■ 85226644353 E‐ mail:hkstc@hkstc org VVebsite:'ぃ Ⅳvwtstc―g:K)up.org
This repon shali not be repmduced unless wlh pnorlwiten apploval iom The Hong Kong Standatts and■ estng Centre Limted.
For Cond‖ ons oflssuan∝ of thisわ a report,please referお the oveneaf o「 Website.
~´~`ヽ
/⌒ ヽ
、「
:ノ

_ _ ■ ‐|― ―・
DateNo.
2015‐ 03-23
HC361381Page I of I
Applicant (Cotle; SUH017) : Succeed Holdings LtdWorkshop l. J, K, L. Superluck Ind Ctr Phase II57 Sha Tsui RdTsuen Wan NT HK
麟)eSCy:量贅;011 01 Sattpie(3) One subnritted sample said to be KAMACRA-100 Cold.Batch / Lot No.:AK0034GPack Size : 4 tablets/boxCountry of Origin : Mumbai
$a&fe( rlReceived Co nEl i titx : In intact original package unelerambient lemperature
Date Sample(s) Receivetl : 201 5-03- l3
Date Testetl : 20:5-03-23
畜1lVCStigatiO菫 l ReqLICSte(1 : Sildella轟 l
Method(s) Usetl H igh Perforrnance Liqu id Chrontatography
Test Rcsult(s) Iest Item(s) 壺《ARttAGlξA-100 Gol懇
Slldcna■ 1 100.3 n:g/tablet
Notc: Average *.eight= 0.50,17 g/tahlet
TAM ng Hang,Ant1lo■y
Authorized SignatoryChemical and Food Deparfinent
For and on behalfofThe Hong Kong Standards and Testing Centrc Ltd.
***** End Of TeSt RepOrt *rrr.*!t
Tho Hong Kong Standards and Testing Centre Liflrited
10 Dai Wang Street, Tai Po lndustrial Estate, Tei Po, N.T., Hgng Kong
Tel: +852 2666 1888 Fax: +852 2664 4353 Email: [email protected] Website: www.stc'group.org
This repori shall not be reproduced unless with prior written approval from The Hong Kong Standards and Testing Centre Lirnrted
For Conditions oi lssuance ol this test report, plea$e r6for to tho overleaf and Website,
ヽ
―1
/∈
■0■ 0
寺一一゛ぅ
環
ヨ
r■
ニ
CIBL

Dtte
No.
Description of Sample(s)
STC Test R22Ω威
C)ne subnlilled sanlple said to be Noxidil′ Ilablets.
Batch/Lot No.:8136019Pack Size:100 tablets/bottle
Count,Of Origin:Thai
Sample(s) Received Condition:
Page 1 ofl
In intact original package underambient temperature
:2016…08-04
:HC393193
Applicant(Code:SUH017): Succeed Holdngs Ltd蚕湾沙[1咀道 1-9競永南貨倉大度 18棲
Date Sample(s) Received z 2016-07-26
I)ate'Ilested 2016‐08-03
Investigation Requested : Minoxidil
Method(s) Used
Test Result(s)
High Performance Liquid Chromatography
TeSt ltem(S) Result
Minoxidil 5.01 mytあ let
* Average weight per tablet :0.72497 g
TAM Hang, AnthonyAuthorized Sign滅 oly
Chelnic」 and Food DepatmentFor and on behalfof
The Hong K:ong Standalds and Testing Centre Ltd.
,rrkrrrr:r End Of TeSt RepOrt ,rr.rrtrrr
The Hong Kong Standards and Testing Centre Limited
10 Dai Wang Street, Tai Po lndustrial Estate, Tai Po, N.T., Hong Kong
Tel: +852 2666 1888 Fax +852 2664 4353 E-mail : [email protected] Website: www.stcgroup.org IThis report shall not be reproduced unless with prior written approval fmm The Hong Kong Standards and Testing Centre Limited.
For Conditions of lssuance of this test aeport, please refer to the overleaf or Website.
ヽヽ´ ・ ■
.´ヽヽ =・・′
=蹴1

ST⊆ Test墜豊QΩ威
I:)ate :2016‐ 08‐04No. :HC393194
Applicant(Code:SUⅡ017): Succeed Holdngs Ltd基湾沙咀道 1‐9競永南貨倉大度 18棲
01ne subnlitted sanple said to be Nbxidil Fo■e Tablets.
Batch/Lot No.:8137025Pack Size:100 tablets/bo■ le
CountW OfOrigin:Thai
Samole(s) Received Condition:
Page 1 of I
In intact original package underambient temperature
Description of Sample(s)
Date Sample(s)Received : 2016‐ 07…26
I)ate■ lested : 2016‐ 08‐03
Investigation Requested : Minoxidil
Method(s) Used
Test Result(s)
High Performance Liquid Chromatography
TeSt ltem(S) Result
4ゝinoxidil 9.68m酢あld* Average weight per tablet : 0.12889
Harg, AnthonyTAMAuthorized Signatory
Chemical and Food DepartmentFor and on behalfof
The Hong Kong Standards and Testing Centre Ltd.
,rrrrr** End of Test Report *rrrrrrrr
The Hong Kong Standards and■ esting Centre Limited
10 DaiVVang Street,■ ai Po industial Estate,Tal Po,N.T,Hong Kong
Tel:+852 2666 1388 Fax:+85226644353 E‐ma‖ :hkstc@hkstcorg VVebsite:wwwst← gЮup.org
This report shall not be reproduced unless with pror writen approvalfrorn The Hong Kong Standards and Testing Centle Lilγ lited.
For Conditons ofissuance ofthis test report,please refer t,the oveneaf or VViebsite.
/´~`
、
/ r―`|
ヽ つ ノヽ /

I)ate i 2012-08-01
No.:IIC305084
Applicant (Code: S{1H017) :
Description of Sample(s)
Plethotl(s) [Jscd
T'est Result(s)
STC Test Report
Succced l― Ioldings I_′ td
7/ド VVol・ kshoP I‐L Supcrltick ind Centrc(Phasc II)
57 Sha′Ilsui Rd
Tsucnヽ″an NT IIK
One submitted sample said to be Finalo.Countr-r.'of Origin : India
S a nr p I c ( il -&eseivrdesn dilr_q!.
Page I of I
In plastic container under ambienttempcrahrre
Date Sample(3)Rece市 ed : 2012-07-20
I)a(e Teslcrl 2012-07‐ 30
lnvcstigationRequested : Finasteritle
The Hong Kong Standards and Tesfing Gentre Ltd.10 Dai Warg Street, Taipo tndustri6l Estate, N.T., hong Koo0
T€l: (852) 2666 1888 Far: (852) 2664 4353 E-m€ll: [email protected] Homaps06: ffi,stc.0roup.orgIhis report shall not be Epmduced unls wlth prior w.itien appmval troa Ths Hong Kong Slendards and Tssting Centfe Ltd.
For Cooditions of lssuan€ of thi6 tsst report, pleas ref€r to lh€ ovedsf or Horupage.
High Pel・ lbl・ Inance Liquid Chl・ omato31・ apll:、 -1)lodc AITay Dctccto「
Test ltenr(s) Finalo
Finastericle 1・00mゴtablCt
Note: Avcrage rvright * 0.15,16gu tablet
KWOK Nga Yan. BAuthorized Signatory
Chemical and Food DeparlurentFor and on behalf of
The Hong Kong Standards and'lesting Centre Ltd
***** En(l ofTest Rcport *****
■0‖ C
v/ヽミト
・
、ξ

″
.
STC Test Re2Qrt
IE)ate :2018-02-28
No.:HC18020495
ApphGIlt(COde3 01311126)
Desc五ption of Sample(s) : One submitted sample said to be LEVITRA 20:mg.
Batch/Lot No.:BXHCIIElExp:09/2019Country of10Hgin:Gerinany
S笙ユI≧1≦2(旦】_1:コ:::」 :VQ`ユニ」〔]Qユ
`菫
11鯉
Page l of l
In intact original package underambient temperature
Date Salmple(s)Received : 2018-02-15
Date Tested
Investigation Requested : Vardenafil
Method(s)Used
T∝t Res■t(s)
High Performance Liquid Chromatography
TeSt ltem(S) Resdt
V″dcna■ 1 19.8 nlノ tablet
Average weight per tablet : 0.18829
Kwok Nga Yan, BerniceAuthorized Signatory
Chemical and Food DepartmentFor and on behalf of
The Hong KOng Stand‐ds and Testing Cen■ c Ltd.
*****End of Tlest R、 eport*****
The Hong Kong Standards and Testing Centre Limited
10 Dai Wang S'treet, Tai Po lndustrial Estate, Tai Po, N.T., Hong Kong
Tel: +852 2666 1888 Fax: +852 2664 4353 E-mail : [email protected] Website: www.stc-group.org
This report shall not be reproduced unless with prior written approval irom The Hong Kong Standards and Testing Centre Limited.
For Conditions ol lssuance ol this test report, please refer to the overleai or Website.
L.
゛ hDI‘」
■日■■■■■■[!―― ■
「
――‐
」 ヒ
: 2018-02-27

:画ISTCDtte
No.:2018-12-11
:HC18111232
Test R◎ p◎『量
Page 1 of 1
Succeed Holdings Linlited
香港新界青衣青衣航運路 36競亜洲物流中心順豊大慶 11棲 2室Applicant (Code :0131 1126) z
Description of Sample(s)
Date Sample(s)Received : 2018-11-30
Date Tested : 2018… 12‐04
Investigation Requested : Minoxidil
Ⅳ【ethOd(S)USed
Test Result(s)
One subnlttcd salnplc said to be Regrow■ l Labs h江 5 60nll
Batch/Lot NQ.:W6810302AExp l)ate:2020/11Country of Origin:USA
Sarnple(s) Received Condition: In intact original package underambient temperature
Liquid Chromatography - Diode Array Detector
TeSt ltem(S) Result
h4inoxidil 5.21%Ⅵ′/v
Density ;0.9893 g/m1
′
餞 ,
ヽ蠍
CHOW HoiYi,A■ ce
Autho五zed Signatory
Chemical and Food DeparffnentFor and on behalf of
The Hong Kong Standards and Testing Centre Ltd.
*****End of Test Report*****
The Hong Kong Standards and Testing Centre Limited
10 Dai Wang Street, Tai Po lndustrial Estate, Tai po, N.T., Hong Kong
Tel: +852 2666 1888 Fax: +852 2664 4353 E-mail [email protected] Website: www.stc.group
This report shall not be reproduced unless with prior written approval from The Hong Kong Standards and Testing Centre Limited.
For Conditions oi lssuance ol this tesl report, please re{er to the overleal or Website

回ISTEl])ate :2019-01-24
No.:HC19010730
Applicant(Code :01311126)
Test RepOF饉
Page 1 of 1
Succeed IIoldings Linlited
香港新界青衣青衣航運路 36琥亜洲物流中心|1瞑豊大度 11模 2室
S笙⊇ 1∝立 壁 CelVed COndi`迎壺 】 In intact original package underambient temperature
Desc五puon of Samメ e(s) : One sublntted sample sdd Ю be Rc『owth Labs M15 60ml.Batch/L∝ No.:W6812173AExp:12/2020Country of O五gin:USA
Date Sample(s) Received : 2019-01-15
Date Tested : 2019¨01‐23
Investigation Requested : Minoxidil
MIethOd(S)USed : Liquid Chromatography - Diode Array Detector
TeSt ltem(S) Result
4ヽinoxidil 16.84%(w/v)
# Density: 1.055891rn1
′
Мr●」ヽヽ
CHOW Hoi Yi, AtceAuthorized Signatory
Chemlc」 and Food DeparmentFor and on bchalf of
The IIong Kong Standards and Te"ing Centre Ltd.
*****E.nd of Test Report*****
The Hong Kong Standards and Testing Centre Limited
10 Dai Wang Street, Tai Po lndustrial Estate, Tai Po, N.T., Hong Kong
Tel: +852 2666 18BB Fax: +852 2664 4353 E-mail [email protected] Website: www.stc.group
This report shall not be reproduced uniess with prior written approval from The Hong Kong Standards and Testino Centre Limited.
For Conditions ol lssuance ol this test report. please reier to the overleai or Website
■ st Result(s)

PAREX PHARMACEUTICALS PVT. LTD
D-1,45, I nd ustria I Area Phase-7.S.A.S. N agar ( Moha I i)-160055 I N DIA
CERTI FICATE O F ANALYSIS
1. Description - Blue coloured diamond shaped coated tablet in blister pack.
2. Avg. weight - 485mg
3. Hardness - 4.9kg/cm2
4. Friability - O.2L%
5. Assay - Each coated tablet contains:
Result
Sildenafilcitrate 99.91mg
Eq.to Sildenafil
claim
100mg
%age
99.9!%
REPORT: The sample received complies/ d€€s-n€+€€mpfy with the prescribed standards of
quality as per IPIBP/USP.
Date: 16/041201,9
Sample : Sildigra 100 tablet Report no. L9-20/L9Mfd by : M/s Parex Pharmaceuticals Pvt.Ltd Ref"no.19Received from -do- Recd. On. t6l04ll9
Batch no.
s-57
D/M
04/2019
D/E
03/2022
Batch size
L,82,O0O
tablets
Sample qty.
100 tablets
RESULT OF ANALYSIS
Reference of protocol applied : FG-LZ
Chemical test
Authorised signatory

PAREX PHARMACEUTICALS PVT. LTD
D-1"45, I nd ustrial Area Phase-7.S.A.S. Naga r (Moha li)-160055 I N DtA
CERTI FICATE OF ANALYSIS
Chemicaltest
1. Description - Blue coloured diamond shaped film coated tablet in blister pack.
2. Avg. weight - 488mg
3. Hardness - 5kg/cm2
4. Friability - 0.2O%
5. Assay - Each film coated tablet contains:
Result
Sildenafil citrate 99.9mg
Eq.to Sildenafil
claim
100mg
%age
99.9%
REPORT: The sample received complies/ d€€s-n€+-€€mp{y with the prescribed standards of
quality as per lPIBP/USP.
I
{hrlzh- -" -I
Date:24/04/2]19 Authorised signatory
Sample : Sildigra 100 Tablet Report no. L9-ZO/2gMfd by : M/s Parex Pharmaceuticals Pvt.Ltd Ref.no.2gReceived from -do- Recd. On. Z4/O4lLg
Batch no.
s-58
D/M
04/201e
D/E
03/2022
Batch size
47,OOO
Tablets
Sample qty.
100 tablets
RESULT OF ANALYSIS
Reference of protocol applied : FG-12

PAREX PHARMACEUTICALS PVT.LTD
D-1,45, lndustrial Area Phase-7.s.A.s.Nagar (Mohali)-160055 I N DtA
CERTI FICATE OF ANALYSIS
Chemical test
1". Description - Blue colored diamond shaped film coated tablet in blister pack.
2. Avg. weight - 501m9
3. Hardness - 5.5kg/cm2
4. Assay - Each film coated tablet contains:
Result claim %age
Sildenafil citrate 49.68mg 50mg 99.37%
Eq.to Sildenafil
REPORT: The sample received complies/ d€es-n€+€€mpJy with the prescribed standards of
quality as per lP/BPIUSP.
rda*",..,--r"
Date: 03/04 /2019 Authorised signatory
Sample : Sildigra 50 Tablet Report no.L9-2OlO4Mfd by : M/s Parex Pharmaceuticals Pvt.Ltd Ref.no.04Received from -do- Tested. On. 03/04/19
Batch no.
ST-34
D/M
04/2ots
D/E
03/2022
Batch size
95,000 Tablets
Sample qty.
100 tablets
RESULT OF ANALYSIS
Reference of protocol applied : FG-11





国STCTest ReQort
Date : 2019-10-02 Page I of I No. : HC19090711
Applicant(Code: 01311126): Succeed Holdings Limited 香港新界青衣青衣航運路36 �虎亜洲物流中心順豊大腹11楼2室
Description of Sample(s) : One submitted sample said to be UC Minoxidil 5mg (FTL). Batch I Lot No.: 9SG04A Exp Date : 2021/8/3 I Country of Origin : Philippines
Sam� In intact original package under ambient temperature
Date Sample(s) Received
Date Tested
Investigation Requested
Method(s) Used
2019-09-20
2019-09-27 to 20 I 9-09-29
Minoxidil
Liquid Chromatography - Diode Array Detector
Test Result(s) Test Item(s)
Minoxidil Average weight per tablet: 127.0mg
Result
5.34 mg/_tablet
CHUNG On Ping, Karen Authorized Signatory
Chemical and Food Department For and on behalf of
The Hong Kong Standards and Testing Centre Ltd.
***** End of Test Report *****
コ誓as
ぐ゚`
.ヽ
々
s
s
.
、\ \`
,
-
3J
“
也’
〖匝E§・ぢ
Note: When a statement of conformity to a specification or standard is provided. the ILAC-G8 Guidance document (and/or !EC Guide 115 in the clcctrotechnical sector) wi l l be adopted as a deciヽion rule for the determination of conformity unless it is inherent in the requested specification or standard, or othe1wise specified in the Report.
The Hong Kong Standards and Testing Centre Limited 10 Dai Wang Street, Tai Po Industrial Estate, Tai Po, N.T., Hong Kong
Tel: +852 2666 1888 Fax: +852 2664 4353 Email: [email protected] Website: www.stc.group This report shall not be reproduced unless with prior written approval from The Hong Kong Standards and Testing Centre Limited.
For Conditions of Issuance of this test report, please refer to the overleaf and Website.






LICENCE NO. GTW17 1; r . z
C -- . --
m!@WiW : - 0 1 / 2 0 2 0 Cerdflcate Na : mOIW1317YW 23 I UC UPEHAFIL ThBlEfS ZQO Ma ~)rb o f u t : 09/0i/2020
m W :CENTURIWREPlhDP€B WT-LTDm mph QQ* : 20 TABLE+( BULK 1 -.w : CEHTURTON REUEDIW WT.LTD, M Q . U G N ~ : L~ b Bawl*. : ,,-,,,, [mm 1 Dm: 31,2019 DlE: $0,2022 Batch Blr? 50610 TABLETS
TEST DATA,
E W P F T C A T I O H
WfBHT OF -20 TB 3
(4) '86.09 X .a 92-44 ?:
: Label Claim
94-30 %
0°C) % - ~10.0 X 3 *
. d ?
sots: I '
1. ~ h s r r r b ~ a m l ~ m l a o d u r n p l e & ~ ~ ~ l e ~ . ~ ~ o l ~ h ~ ~ i . R n b d ~ ~ - 2. T d liability of our i n ~ h t e is limited to the lovo id amotmt of this report
1 3. ~ ~ s ~ i s a o t t o b e ~ w h o ~ a r i a p r r r t a n d ~ ~ d a s ~ w i ~ i n ~ ~ ~ o f ~ w , a n d & d n o t b s I u s w i i n a n y ~ m s d i a w i t h o u t o u r ~ ~ ~ $ i o n i n w r i t i o g .
4. Sample drawn & submitted by the party for andpin d m &hemhe stared. I.-- -, . 5, ~ m p m t ~ a a t i n f e r ~ t y 0 f t h e ~ S a b j c c t t o ~ J ~ c t i ~ - .
; 6. T B e s l m p l ~ w o u l d b ~ a f t a 3 . o n b m o m t h ~ t h e d a t e d ~ ~