Evaluation of Nontumorous Tissue Damage by Transcatheter … · 11Sex(M/F)5/111/0Age (yr)59.83...
Transcript of Evaluation of Nontumorous Tissue Damage by Transcatheter … · 11Sex(M/F)5/111/0Age (yr)59.83...
-
(CANCER RESEARCH 51, 5667-5671. October 15. 1991]
Evaluation of Nontumorous Tissue Damage by Transcatheter Arterial Embolizationfor Hepatocellular Carcinoma
Khaleque Newaz Khan, Keisuke Nakata, Yukio Kusumoto, Masayoshi Shima, Nobuko Ishii,Toshihiko Koji, and Shigenobu Nagataki1
First Department of internal Medicine, School ofMedicine [K. N. K., K. N., Y. K., M.S., S. N.J, and Health Center fN. I., T. K.J, Nagasaki University, Nagasaki 852, Japan
ABSTRACT
The serial changes in serum hepatic enzyme activities by transcatheterarterial embolization (TAE) were analyzed in 17 patients with hepato-cellular carcinoma to estimate the contribution to the value by the damageof tumor or nontumorous hepatic cells. The serum levels of relativelytumor-specific fructose 1,6-diphosphate (FDP) aldolase were elevatedafter TAE in the cases of both superselective and nonsuperselective TAEthat were performed from the segmental and the nonsegmental hepaticartery, respectively, but we found the marked elevation of FDP aldolasein the cases of the superselective TAE. In contrast, the non-tumor-specific fructose 1-phosphate (F1P) aldolase was markedly elevated onlyin the cases of nonsuperselective TAE. The total amount of FDP aldolasereleased by TAE correlated significantly with the integrated tumor tissuevolume (/' < 0.005), whereas the total amount of F1P aldolase output
correlated significantly with the integrated nontumorous tissue volume(/' < 0.005) as defined by lipiodol accumulation on computerized tomog
raphy scan. The consequent changes in the total nontumorous livervolumes after TAE were also analyzed by the follow-up computerizedtomography scan. The nonsuperselective TAE caused the significant totalnontumorous liver atrophy when compared with the superselective TAE.The progression of the total nontumorous liver atrophy correlated significantly with F1P aldolase output by TAE (P < 0.001) but not with FDPaldolase output. These results suggest that the outputs of FDP and F1Paldolase are useful to estimate the degree of the tumorous and nontumorous tissue damage by TAE, respectively, and F1P aldolase outputcan be used to predict the progression of liver atrophy caused by TAE.
INTRODUCTION
TAE2 causes the effective tumor necrosis of hepatic neoplasms, both primary and metastatic, by causing tumor devas-cularization (1). The concept of treating liver tumors by theinterruption of their arterial supply was first suggested byMarkowitz (2) in 1952. TAE alone or combined with chemo-therapeutic agents has been considered to be an effective palliative treatment in patients with unresectable hepatic tumors (1,3, 4). The previous study (5) showed that the serum enzymechanges by TAE could be used to estimate the size of thenecrotic mass and to evaluate the effectiveness of the treatment.However, enzyme changes did not analyze the nontumoroustissue damage by TAE which could be an indicator of theadverse effect of TAE in patients with HCC. In clinical practice,we found that TAE impaired liver function, as manifested byelevation of serum transaminase levels or decrease in serumalbumin and cholinesterase levels, sometimes resulting in astate of mild to moderate hepatic failure. Although the changes
Received 4/16/91; accepted 7/30/91.The costs of publication of this article were defrayed in part by the payment
of page charges. This article must therefore be hereby marked advertisement inaccordance with 18 U.S.C. Section 1734 solely to indicate this fact.
' To whom requests for reprints should be addressed, at First Department ofInternal Medicine, School of Medicine, Nagasaki University, 7-1 SakamotoMachi, Nagasaki 852, Japan.
2The abbreviations used are: TAE, transcatheter arterial embolization; FDP,fructose 1,6-diphosphate; F IP. fructose 1-phosphate; HCC. hepatocellular carcinoma; CT, computerized tomography; AFP. «-fetoprotein; AST, aspartate ami-notransferase; ALT, alanine aminotransferase.
in the liver function after TAE appear to be responsible for notonly the tumor tissue necrosis but also the damage of nontumorous liver tissue caused by TAE, there is no report concernedwith the quantitative analysis of the nontumorous liver tissuedamage by TAE to our knowledge.
The purpose of this study is to estimate the serial changes inserum enzyme activities released from the damaged tumor ornontumorous tissue by TAE and to measure the contributionto the value by the damage of tumorous or nontumorous hepaticcells. We mainly selected cytosolic FDP and F1P aldolaseisoenzymes, because HCC has a much greater concentration ofFDP aldolase and a much less concentration of F1P aldolasethan nontumorous liver tissue (6, 7). We also evaluated theconsequent changes in the total nontumorous liver volumes byTAE and compared the results with F1P or FDP aldolaseoutput released by TAE.
PATIENTS AND METHODS
Patients. The first TAE was performed in all 22 patients admitted toour hospital during the period from March 1989 to June 1990. Amongthem, 5 patients with the diffuse type of HCC were excluded from thisstudy because of the difficulties in calculating the tumor or the nontumorous volume by CT scan in these patients. Consequently, 17 patientswith informed consent were used in this study. All of the patients hadHCC associated with liver cirrhosis. There were 16 men and onewoman, aged from 43 to 74 yr [61.9 ±8.8 yr old (SD)]. The diagnosisof HCC was made clinically by the characteristic appearance of ultrasonic tomography, CT scan, and angiography. Liver cirrhosis wasdiagnosed by laparoscopy and liver biopsy. Twelve patients had anAFP-producing tumor with serum AFP levels more than 50 ng/ml, andthe other five patients had serum AFP levels less than 20 ng/ml. Thesuperselective TAE from the second or third branch of the hepaticartery was performed in 6 patients and the nonsuperselective TAE fromthe proper, right, or left hepatic artery in 11 patients. As shown inTable 1, the mean age and the tumor size were not different betweenthe two groups. The procedure of TAE was described elsewhere (4).Lipiodol was used together with Spongel to calculate the integratedvolumes of tumorous and nontumorous tissues.
Sera were collected before and 1, 2, 4, 8, 12, and 24 h after TAE andon Days 2 through 10 when enzyme activities returned to the pretreatment levels. The sera were stored at -20°Cuntil use.
Enzyme Assays. We measured the FDP and F1P aldolase activitiesby the colorimetrie methods that served as the basis for the proceduredescribed in the Sigma diagnostic kit. Serum AST and ALT activitieswere assayed by an automatic analyzer (Hitachi Model 736; Hitachi,Tokyo, Japan). Serum AFP concentrations were also measured by acommercially available radioimmunoassay kit (Dainabot, Tokyo, Japan.
Model for Calculation of Cumulative Amounts of Enzyme Released.The model for calculation of the cumulative amounts of enzyme released from the damaged tumorous or nontumorous tissue is based ona modification of the method of Sobel et al. (8) and Norris et al. (9)originally designed for the measurement of the total creatine phospho-kinase release into the circulation by myocardial infarction.
Calculation of the Integrated Volume of Tumorous and NontumorousTissue by the Followed CT Scan. Serial CT scans were taken with a
5667
on March 31, 2021. © 1991 American Association for Cancer Research.cancerres.aacrjournals.org Downloaded from
http://cancerres.aacrjournals.org/
-
NONTUMOROUS TISSUE DAMAGE BY TAE FOR HCC
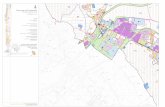
Fig. 1. Distribution of lipiodol in tumorous and nontumorous tissue as shown by CT scan on the third day (A and C) and 2 wk (B and D) after TAE. A case of thesuperselective TAE (A, B) and a case of the nonsuperselective TAE (C, D) is represented.
Table 1 Comparison of ages and tumor sizes between the cases of thesuperselective and the nonsuperselective TAE
Type ofTAESuperselective
NonsuperselectiveNo.
ofpatients6
11Sex(M/F)5/111/0Age
(yr)59.83±8.4"
63.09 ±9.2Tumor
size(cm)NS»
5.41 ±2.14.31 ±2.1NS
" Mean ±SD.* NS, not significant (P > 0.05).
the natural course of liver atrophy in 26 cirrhotic patients without HCCwho were admitted to our hospital during the same period. CT scanswere taken just before and 1 mo, 3 mo, and 6 mo after confirming thediagnosis of liver cirrhosis by laparoscopy and liver biopsy. There were18 men and 8 women aged from 39 to 74 yr (56.4 ±10.5 yr old).
Statistical Analysis. All values were expressed as the mean ±SD.Statistical analyses were carried out by the x2 test or the Student t test.
Somatom 2 (Siemens AG, Erlangen, Federal Republic of Germany) at1-cm intervals. The integrated tumor area of each slice (S, cm2) was
outlined as an area of accumulated lipiodol 2 wk after TAE, and theintegrated volume of the inner tumor mass was calculated by planimeter(Aloka, Tokyo, Japan) with the following formula (5).
(cm3) = (5, + + S„)1/C2
where C (scale ratio) is constant, and VTis the integrated tumor volume.In the same way, we outlined the area of accumulated lipiodol on the
third day CT scan in which lipiodol was distributed in both tumorousand nontumorous tissue according to the distributing pattern of thevessel where the tip of the catheter was inserted (Fig. 1). The integratednontumorous tissue volume was calculated by subtracting the volumeof tumor mass from the total integrated volume.
Serial Changes in the Total Nontumorous Liver Volumes after TAE.Since the accumulated lipiodol in the integrated nontumorous livertissue disappears time dependently, we calculated the total nontumorous liver volume before and 1 mo, 3 mo, and 6 mo after TAE bysubtracting the tumor volume from the total liver volume as describedbefore. In addition to the 17 representative patients, we also analyzed
RESULTS
Serial Changes in Serum FDP and F1P Aldolase Activities byTAE. The serial changes in serum FDP and F1P aldolaseactivities by TAE in 17 representative cases are shown in Fig.2. In most of the cases, the enzyme levels began to rise 4 to 8h after TAE, peaked at 24 to 48 h, and returned to the preem-bolization level within 7 to 10 days. Nonsuperselective TAEcaused marked elevation of the serum F1P aldolase level,whereas the serum F1P aldolase level was almost unchanged inthe case of superselective TAE (Fig. 2, right). In contrast, FDPaldolase activities were markedly elevated in the superselectiveTAE, but relatively less elevation was observed in the nonsuperselective TAE (Fig. 2, ¡eft).
Correlation of the Total FDP or FI P Aldolase Output Releasedby TAE with the Integrated Tumor or Nontumorous Volume.The integrated nontumorous tissue volumes in the cases of thenonsuperselective and superselective TAE were 268.2 ±79.8cm3 and 91.8 ±48.1 cm3, respectively. The difference wasstatistically significant (P < 0.001). The integrated tumor vol-
5668
on March 31, 2021. © 1991 American Association for Cancer Research.cancerres.aacrjournals.org Downloaded from
http://cancerres.aacrjournals.org/
-
NONTUMOROUS TISSUE DAMAGE BY TAE FOR HOC
FDP ALDOLASE
Fig. 2. Serial changes in the serum FDPand F1P aldolase levels after TAE. The thicklines and the thin lines represent the cases ofnonsuperselective TAE and superselectiveTAE, respectively.
oo
tBEFORE
24h"2d
TIME AFTER EMBOLIZATION
F1P ALDOLASE
on 24h 2 0.05).
20 40 60 80
FDP ALDOLASE OUTPUT (UA.)
ÃŽ1Õ1Jf400-300-200-100-0.•••¿�
•¿�
•¿�•¿�•¿�•¿�*•¿�••
•¿�•¿�•
r=0.4W•
p>0.05
20 40 60 80
FDP ALDOLASE OUTPUT (UA.)
100
Fig. 4. Correlation of the total F1P aldolase output with the integrated tumor or nontumorous volume. F1P aldolase output correlated significantly with the nontumorous volume (P < 0.005) but not with the tumorvolume (P > 0.05).
500
"00
300-
r=0.36Jp>0.05
500
10 20 30 50
i 400-
3 SOO-
ft 200-
f »:o
F1P ALDOLASE OUTPUT (UA.)
10 20 30 40
F1P ALDOLASE OUTPUT (UA.)
50
umes were not different between the two groups (134.7 ±105.3versus 205.2 ±176.9 cm3, P = 0.31). The total enzyme output
of FDP aldolase correlated significantly with the tumor volumebut not with the nontumorous volume (Fig. 3). In contrast, thetotal enzyme output of F1P aldolase correlated significantlywith the nontumorous volume but not with the tumor volume(Fig. 4). When the FDP/F1P aldolase output ratio in thesuperselective TAE was compared with that in the nonsuperselective TAE, the ratio in the superselective TAE was significantly greater than that in the nonsuperselective TAE (P <0.005; Table 2).
Correlation of the Total ALT or AST Output Released byTAE with the Integrated Tumor or Nontumorous Volume. Serumlevels of ALT and AST began to rise 4 to 8 h and returned topretreatment levels within 7 to 10 days after TAE. When thetotal outputs of these enzymes were compared with the tumoror the nontumorous volume, the total ALT output correlated
Table 2 FDP/FÃŒPaldolase outpul ratio in superselective andnonsuperselective TAE
Superselective TAE(n = 6)
Nonsuperselective TAE(n= II)
FDP/FIP ratio 5.0 ±1.0°* 2.6 ±1.0
Mean ±SD.< 0.005.
significantly with the nontumorous volume (P < 0.05) but notwith the tumor volume. The total AST output correlated withboth the tumor (P < 0.05) and the nontumorous volume (P <0.005; Fig. 5).
Serial Changes in Serum AFP Levels after TAE. Serialchanges in serum AFP levels after TAE were determined in 12patients with serum AFP concentrations more than 50 ng/mlbefore TAE and expressed as a percentage of the value beforeTAE (Fig. 6). Serum AFP levels rose to their highest level
5669
on March 31, 2021. © 1991 American Association for Cancer Research.cancerres.aacrjournals.org Downloaded from
http://cancerres.aacrjournals.org/
-
NONTUMOROUS TISSUE DAMAGE BY TAE FOR HCC
Fig. 5. Relationship between total ALT orAST output and the integrated tumor or non-tumorous tissue volume after TAE. ALT output correlated with nontumorous volume (P <0.05) but not with the tumor volume, whereasAST output correlated with both the tumorand the nontumorous volume (P < 0.05 and P< 0.005, respectively).
500
1 400-1
§ 300-
OÅ’ 200-
¡5 100-
500
400 -
r=0.589
p-0.05
800 1000 1200
TOTAL AST OUTPUT (U/L)
r-0.192p-0.05
100 200 300 400 500
TOTAL ALT OUTPUT (U/L)
600
500
a 400iB 300
200-
100-
I
200 400 600 800 1000 1200
TOTAL AST OUTPUT (U/L)
100 200 300 400 500
TOTAL ALT OUTPUT (U/L)
tBEFORE
TAE
24h 2d 5d
TIME AFTER EMBOLIZATION
Fig. 6. Serial changes in serum AFP levels after TAE. The thick lines and thethin lines represent the cases of nonsuperselective TAE and superselective TAE,respectively. The values are expressed as a percentage of the pretreatment values.
within 6 h after TAE and gradually decreased thereafter. Insuperselective TAE, the serum AFP level decreased to less thannearly a half of the pretreatment values on the seventh day,whereas it did not in the nonsuperselective TAE.
Total Nontumorous Liver Atrophy after TAE. The total nontumorous liver volumes were estimated by the follow-up CTscans 1, 3, and 6 mo after TAE and compared with the naturalcourse of liver atrophy in 26 cirrhotic patients without HCC(Table 3). The nonsuperselective TAE resulted in significanttotal nontumorous liver atrophy, when compared with thesuperselective TAE and the natural course of liver cirrhosis.The degree of total nontumorous liver atrophy caused by the
superselective TAE was not different from liver atrophy in thenatural process of liver cirrhosis.
Relation of the Percentage of the Total Nontumorous LiverAtrophy with the Total FDP or F1P Aldolase Output Releasedby TAE. Since FDP and F1P aldolase were mainly releasedfrom the tumor and the nontumorous tissue of the liver, respectively, we analyzed the correlation of the percentage of thetotal nontumorous liver atrophy 1 mo after TAE with the totalof these enzyme outputs (Fig. 7). The percentage of liver atrophy correlated significantly with the total F1P aldolase output(r = 0.867, P < 0.001) but not with the total FDP aldolaseoutput (r = 0.316, P > 0.05).
DISCUSSION
Although three fourths of the total hepatic blood supply areprovided by portal vein, the hepatic artery provides 40 to 60%of the oxygen supply to the liver (10). Popper et al. (il) reportedthat the portal vein plays only a secondary role as a source ofoxygen supply. The limited amount of oxygen supply to theliver by the portal blood flow is not sufficient to prevent livernecrosis by the interruption of the hepatic arterial perfusion(11). To date, several reports revealed the various complicationsof TAE (12-16). However, the quantitative analysis of thenontumorous liver damage by TAE in patients with HCC hasnot been reported previously. In this study, we evaluated theadverse effect of TAE on nontumorous liver tissue.
Although there are several factors in TAE, as for example,size of the tumor, position of the catheter tip, appropriatefeeding artery, size of the Spongel particle, volume of theSpongel material, and blood flow of the feeding vessels, weconsidered the position of the catheter tip, the appropriatefeeding vessel, and the fixed size and volume of the Spongelmaterial for our study. We found marked elevation of serumFDP aldolase activities in the superselective TAE and relativelyless elevation of serum FDP aldolase activities in the nonsuperselective TAE. These results coincided with the more significant decline of serum levels of AFP in the cases of superselective TAE than that in the nonsuperselective TAE. In contrast,
5670
on March 31, 2021. © 1991 American Association for Cancer Research.cancerres.aacrjournals.org Downloaded from
http://cancerres.aacrjournals.org/
-
NONTUMOROUS TISSUE DAMAGE BY TAE FOR HCC
Table 3 Percentage of total nontumorous liver atrophy after TAE in patients with HCCStatistics were analyzed by the \2 test.
Type ofTAESuperselective
NonsuperselectiveLiver cirrhosis (control)No.
ofpatients6
1126~1
mo ~3 mo ~6moflI.9U
±1.8 -i11.93 ±7.0 =4*
1.23 + 0.7 _T19
±2.3-iNS'15.42 ±12.5=]'
3.4 ±1.3 J4.4
±J.8 -INS 17.66 ±10.7=¿'
4.2 ±1.7 _TNS
" Mean ±SD.V-cO.05.c NS, not significant (P > 0.05)."P< 0.001.
Fig. 7. Correlation of the percentage of thetotal nontumorous liver atrophy after TAEwith the total FDP or F1P aldolase outputreleased by TAE. The total nontumorous liveratrophy correlated significantly with the F1Paldolase output (P < 0.001) but not with theFDP aldolase output (P > 0.05).
30
> 10 -
r=0.867p-0.001
30
f 0316
p 0.159
10 20 30 40
F1P ALDOLASE OUTPUT (U/L)
50 20 40 60 80 100
FDP ALDOLASE OUTPUT (U/L)
serum F1P aldolase activities were significantly elevated in thecases of the nonsuperselective TAE. We also found that thetotal enzyme outputs of FDP aldolase and F1P aldolase correlated significantly with the tumorous and nontumorous tissuevolumes, respectively, and that the FDP/F1P aldolase outputratio in the superselective TAE was significantly greater thanthat in the nonsuperselective TAE. These results indicate thatthe nonsuperselective TAE causes the increase in the release ofF1P aldolase from the damaged nontumorous liver tissue. Infact, the occlusion of segmental tumor feeding vessels by embolie materials is more selective than that of nonsegmentalvessels where the embolie materials are distributed in a scatteredfashion and entered into the intrahepatic branches of the othermajor hepatic arteries.
Unlike the previous report (5), we did not find correlation ofthe total ALT output with tumor tissue volume. The total ASToutput correlated with not only the tumor but also the nontumorous tissue volume. We also found that the serum AST andALT activities after TAE were higher in the nonsuperselectiveTAE than those in the superselective TAE. These results suggest that the elevation of these enzymes by TAE is responsiblefor an ischemie or hypoxic damage of the nontumorous tissuerather than the tumorous tissue necrosis.
The nontumorous liver damage caused by TAE was followedby the consequent development of the nontumorous liver atrophy, as were 26 control cirrhotic patients without HCC. Therewas no difference of liver atrophy between the superselectiveTAE and the natural course of liver cirrhosis. In contrast, thenontumorous liver atrophy caused by the nonsuperselectiveTAE was more progressive than that caused by the superselective TAE and the natural course of liver cirrhosis. This progression of liver atrophy after proximal TAE is mainly responsible for the nontumorous parenchymal damage, because theprogression of the nontumorous liver atrophy correlated significantly with the total F1P aldolase output released by TAE.
Thus, we reported here, nonsuperselective TAE causes significant nontumorous liver damage, impairing the reservedfunction of the liver, resulting in the subsequent developmentof liver atrophy after TAE. We also suggest that the measurement of serum FDP and F1P aldolase activities can be used to
evaluate the degree of tumor necrosis and nontumorous tissuedamage by TAE, respectively, and the elevation of serum F1Paldolase levels after TAE is useful to predict the subsequentdevelopment of liver atrophy.
REFERENCES
1. Chuang, V. P., and Wallou, S. Hepatic artery embolization in the treatmentof hepatic neoplasms. Radiology, 140: 51-58, 1981.
2. Markowitz, J. The hepatic artery. Surg. Gynecol. Obstet., 95:644-646,1952.3. Okamura, J., Horikawa, S., Fujiiyama, T., Mondem, M., Kanbayashi, '..
Sikuhara, ()., Sakurai, M., Kumda, C., Nakamura, II, and Kosagi, G. Anappraisal of transcatheter arterial embolization combined with transcatheterarterial infusion of chemotherapeutic agents for hepatic malignancies. WorldJ. Surg., 6: 352-357, 1982.
4. Yamada, R., Sato, M., Kawabata, M., Nakatsuka, II.. Nakamura, K , andTakashima, S. Hepatic artery embolization in 120 patients with unresectablehepatoma. Radiology, 148: 397-401, 1983.
5. Yoshikawa, C., Shimojo, N., Naka, K., Akai. T., Okuda, K., Kaminou, T.,Yamada, T., and Nakatsuka, H. Changes in serum enzyme activity aftertranscatheter arteria! embolization for hepatic neoplasm. Clin. Biochem., 20:435-440, 1987.
6. Asaka, M., and Alpert, E. Subunit-specific radioimmunoassay for aldolaseA, B, and C subunits: clinical significance. Ann. NY Acad. Sci., 417: 359-367, 1983.
7. Asaka, M., Miyazaki, T., Hollinger, F. B., and Alpert, E. Human aldolase Bserum levels: a marker of liver injury. Hepatology. 4: 531-535. 1984.
8. Sobel, B. E., Bresnahan. G. F.. Shell, W. E., and Yoder, R. D. Estimation ofthe infarcìsize in man and its relation to prognosis. Circulation, 46: 640-648, 1972.
9. Norris, R. M., Whitlock. R. M. L., and Small, C. W. Clinical measurementof myocardial infarcìsize. Modification of a method for the estimation oftotal creatine phosphokinase release after myocardial infarction. Circulation,5/: 612-620, 1975.
10. Wyngaarden, J. B., and Smith, L. H. (eds.). CECIL, Text Book of Medicine,Ed. 18, p. 847. Philadelphia: W. B. Saunders Company. 1988.
11. Popper. H. L., Jefferson, N. C., and Necheles, H. Interruption of all arterialblood supply to the liver not compatible with life. Am. J. Surg., 84: 429-431, 1952.
12. Trojanowski, J. O., Harrist, T. J., Athanosoulis, C. A., and Greenfield, A. J.Hepatic and splenic infarction: complications of therapeutic transcatheterembolization. Am. J. Surg., 139: 272-277, 1980.
13. Jacob, E. T., Shapira, Z., Morag, B., and Rubenstein, Z. Hepatic infarctionand gallbladder necrosis complicating arterial embolization for bleedingduodenal ulcer. Dig. Dis. Sci., 24: 482-484, 1979.
14. Bradley, E. L., and Goldman, M. L. Gastric infarction after therapeuticembolization. Surgery (St. Louis), 79:421-424, 1976.
15. Shapiro, N.. Brandi, L., Sprayregen, S., Mitsudo. S., and Glotzer, P. Duodenal infarction after therapeutic Gelfoam embolization of a bleeding duodenal ulcer. Gastroenterology, 80: 176-180, 1981.
16. Miller, R. E., Baer, J. W., Nizin, J. S., and Pascal, R. R. Hemorrhagicpancreatitis: a complication of transcatheter embolization treated successfullyby total pancreatectomy. Am. J. Surg., 149: 802-808, 1985.
5671
on March 31, 2021. © 1991 American Association for Cancer Research.cancerres.aacrjournals.org Downloaded from
http://cancerres.aacrjournals.org/
-
1991;51:5667-5671. Cancer Res Khaleque Newaz Khan, Keisuke Nakata, Yukio Kusumoto, et al. Arterial Embolization for Hepatocellular CarcinomaEvaluation of Nontumorous Tissue Damage by Transcatheter
Updated version
http://cancerres.aacrjournals.org/content/51/20/5667
Access the most recent version of this article at:
E-mail alerts related to this article or journal.Sign up to receive free email-alerts
Subscriptions
Reprints and
To order reprints of this article or to subscribe to the journal, contact the AACR Publications
Permissions
Rightslink site. Click on "Request Permissions" which will take you to the Copyright Clearance Center's (CCC)
.http://cancerres.aacrjournals.org/content/51/20/5667To request permission to re-use all or part of this article, use this link
on March 31, 2021. © 1991 American Association for Cancer Research.cancerres.aacrjournals.org Downloaded from
http://cancerres.aacrjournals.org/content/51/20/5667http://cancerres.aacrjournals.org/cgi/alertsmailto:[email protected]://cancerres.aacrjournals.org/content/51/20/5667http://cancerres.aacrjournals.org/