Diagnostic Imaging of Lung Cancer by MRI
Transcript of Diagnostic Imaging of Lung Cancer by MRI

Diagnostic Imaging of Lung Cancer by MRI
S. Bredow1, D. Esparza1, K. K. Divine1, and D. O. Kuethe2
1Lovelace Respiratory Research Institute, Albuquerque, NM; 2New Mexico Resonance,Albuquerque, NM
Lung cancer is the leading cause of cancer-related death in the UnitedStates and is reaching epidemic proportions worldwide. Chemotherapy astreatment has proven largely ineffective, and presently �85% of lung cancerpatients will succumb to the disease. Early detection offers the best chancein reducing mortality and increasing patient survival.
X-ray computed tomography (CT), has made it fairly easy to identifysmall solitary pulmonary nodules (SPNs) in humans—small masses in thelung with roughly a 50/50 chance of being malignant. However, findingsmall SPNs with CT creates a serious dilemma in that it can’t distinguishbenign from malignant tissue in SPNs � 1 cm. Presently, two alternativesexist both with serious deficiencies: 1st, immediate surgical removal of SPNswhich may expose the patient to unnecessary risk, discomfort, and expenseand 2nd, confirming malignancy by allowing nodules to grow to �1 cm indiameter thus, increasing both the probability of a poor prognosis andpatient anxiety. Positron emission tomography (PET) is a non-invasive ima-ging technique that can effectively discriminate benign from malignantnodules, provided the nodules are equal to or greater than 1 cm in diameter.Our goal is to develop a Magnetic Resonance Imaging (MRI) technique cap-able of both imaging smaller (sub-centimeter) nodules and discriminatingbenign from malignant masses. This will enable earlier detection andincrease the odds of successful intervention in human lung cancer.
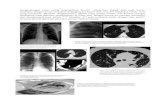
Although MRI is commonly used to detect cancer, imaging the lung withMR can be difficult due to the tissue’s low abundance of hydrogen in theform of water. Our experience suggests MRI of the lung is not as difficultas is commonly perceived, and great potential for advancements in this areaexist. We have successfully used conventional tissue MRI for the imaging oflung cancer in a nude rat model. Animals were intratracheally implantedwith human Calu-6 lung cancer cells and imaged between weeks 5 and 7upon instillation. Results show 1) that lung tumors were clearly visible at thisstage (as corroborated by gross pathology) and 2) the ability to trackchanges in tumor growth over time in the same animal.
85
Experimental Lung Research, 30:85–130, 2004Copyright # Taylor & Francis Inc.ISSN: 0190-2148 print/1521–0499 onlineDOI: 10.1080/01902140490481359
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

Hyperpolarized Gases in Imaging COPD
J. R. BrookemanSchool of Medicine, University of Virginia, Charlottesville, VA
Introduction
A development which began life as an exotic laser-polarized spin targetfor high-energy physics experiments has provided a remarkable new techni-que for imaging the human lungs. The high-energy physics experimentsneeded a target of highly polarized helium-3 (He-3) atoms in which a sub-stantial number of the nuclear spins of the He-3 atoms were aligned inthe same direction. When this many nuclear spins are aligned, they createa very large nuclear magnetization, which to a Magnetic Resonance Imaging(MRI) scanner, means that a large signal is available to form an image fromjust a limited amount of hyperpolarized He-3 gas. With this technique anMR image representing a 1-cm coronal section of the human lungs canbe acquired in about 1 second during a short breath-hold after inhalationof one liter of gas containing just 350 ml of polarized He-3 mixed with nitro-gen. The nuclear magnetization achieved by the laser optical-pumping pro-cess is about 100,000-fold stronger than that available with conventionalmagnetic fields. Allowing for the differential in gas/tissue densities thiscan still provide a signal gain of about 100-fold compared to conventionalMRI of the protons in tissue water molecules. Several medical imaging appli-cations in which the noble gases, helium-3 or xenon-129, can safely be intro-duced into body cavities such as the lungs or sinuses are now underinvestigation at various research centers world-wide.
Applications in the Lung
Most of the early investigations employed a conventional gradient-echomethod for acquiring the MR images of the lungs, whereby approximatelyfifteen 1-cm coronal sections are obtained during a breath-hold period of10–15 seconds. Under these conditions, the intensity in the MR image repre-sents the density of the gas, a dilute mixture of He-3 in nitrogen, filling theventilated lung air spaces. This is termed a ventilation image, where theabsence of signal in any part of the normally-ventilated lung structure iscalled a ventilation defect. These defects may be due to any one of severalfactors, for example mucus plugging or a bronchospasm that restricts thegas flow to distal segments of the lung. Since the first human lung studieswith He-3 in healthy volunteers were reported in 1996, there has been a
86 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

steady progression of new applications of this imaging technique to variouslung pathologies. The initial focus was predominantly on heavy smokers andsubjects with COPD [1, 2], where the MR images show a pattern of charac-teristic ventilation defects. Subsequent investigations have included cysticfibrosis [3] and asthma [4], where similar patterns of ventilation defectswere observed, but with certain features correlated with the underlying dis-eases. In studies at the University of Virginia, which now include over 450human subjects, we have observed that the ventilation defects seen inasthma and cystic fibrosis can be seen to partially or fully resolve in responseto interventions such as administration of a bronchodilator or physical ther-apy [5]. Similarly in subjects with particular sensitivities to a challenge, suchas methacholine or physical exercise, the number and extent of ventilationdefects are observed to increase [6].
New Directions for Imaging COPD
One of the hallmarks for the success of MRI as a diagnostic tool has beenits ability to obtain different types of images providing different tissue infor-mation. These include proton density-, T1- and T2-weighted images, andmore recently MR angiography and diffusion-weighted images for studyingvascular structures and stroke. A similar pattern is emerging in hyperpolar-ized-gas MR imaging. At the University of Virginia we have begun to explorethe possible clinical applications of two new He-3 imaging techniques:dynamic imaging [7] and Apparent Diffusion Coefficient (ADC) imaging [8].The initial results from these studies are very promising as they appear toprovide unique functional and structural information about the lungs notcurrently available by other methods. Cine image sets, with a frame rateof 100 images per second, depicting inhalation and exhalation are possiblewith the dynamic technique, which can dramatically capture the unique dif-ferential gas-flow dynamics in emphysema patients: the technique is able todistinguish regions of the lung that are slow to fill and empty or subject toair-trapping. The ADC technique, which provides regional maps giving ameasure of the restricted diffusion of the He-3 atoms in the lung relatedto alveolar size, already shows promise for providing an early indication ofthe onset of emphysema, when therapeutic intervention might be most ben-eficial. In a recent side-by-side comparison study of CT versus hyperpolar-ized He-3 in identified smokers, MR imaging with He-3 demonstratedmore extensive emphysematous change than CT suggesting a greater sensi-tivity, but the specificity is as yet unknown.
Helium-3 versus Xenon-129
Unlike helium, xenon has a relatively high solubility in blood and tissue,and a large NMR chemical shift, which causes a significant change in the MR
Poster Abstracts 87
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

frequency when xenon atoms diffuse from the alveolar space into the tissuestructures of the lungs [9]. This may permit the acquisition of separate MRimages to distinguish lung airspaces from the tissue and blood compartments.Recent Xe-128 MRI animal studies [10] also hold out the promise of obtain-ing regional maps of gas exchange in the lungs. Current technical challengeslimit the polarization levels obtainable with Xe-129 compared to thatachieved with He-3, and this limits the present wider application of xenonto human studies. However continued improvements in the polarizationtechnology can be expected as interest in this new imaging technology grows.
References
1. Kauczor HU, Ebert M, Kreitner KF, et al. Imaging of the lungs using 3He MRI: Preliminary clinicalexperience in 18 patients with and without lung disease. J Magn Reson Imaging 1997; 7:538–543.
2. de Lange EE, Mugler III JP, Brookeman JR, et al. Lung air spaces: MR imaging evaluation with hyper-polarized 3He gas. Radiology 1999; 210:851–857.
3. Donnelly LF, MacFall JR, McAdams HP, et al. Cystic fibrosis: Combined hyperpolarized 3He-en-hanced and conventional proton MR imaging in the lung—Preliminary observations. Radiology1999; 212:885–889.
4. Altes TA, Powers PL, Knight-Scott J, et al. Hyperpolarized 3He MR lung ventilation imaging in asth-matics: Preliminary findings. J Magn Reson Imaging 2001; 13:378–384.
5. Salerno M, Altes TA, Mugler III JP, et al. Hyperpolarized noble gas MR imaging of the lung: Emergingclinical applications. Eur J Radiol 2001; 40:33–44.
6. Samee S, Altes T, Powers P, Knight-Scott J, Mugler III JP, Ciambotti JM, Alford B, Brookeman JR, deLange EE, Platts-Mills TAE. Imaging the lungs in asthmatic patients by using hyperpolarized helium-3MRI: Assessment of response to methacholine and exercise challenge. J Allergy Clin Immunol 2003;111:1205–1211.
7. Salerno M, Altes TA, Brookeman JR, et al. Dynamic spiral MRI of pulmonary gas flow using hyper-polarized 3He: Preliminary studies in healthy and diseased lungs. Magn Reson Med 2001; 46:667–677.
8. Salerno M, de Lange EE, Altes TA, Truwit J, Brookeman JR, Mugler III JP. Emphysema: Hyperpolar-ized 3He diffusion MRI of the lungs: Comparison with pulmonary function tests—Initial experience.Radiology 2001; 222:252–260.
9. Mugler III JP, Driehuys B, Brookeman JR, et al. MR imaging and spectroscopy using hyperpolarized129Xe gas: Preliminary human results. Magn Reson Med 1997; 37:809–815.
10. Ruppert K, Brookeman JR, Hagspiel KD, Mugler III JP. Probing lung physiology with xenon polariza-tion transfer contrast (XTC). Magn Reson Med 2000; 44:349–357. For more information see: http://imaging.med.virginia.edu/hyperpolarized/index.htm
Skeletal Muscle Dysfunction in COPD
R. CasaburiHarbor–UCLA Research and Education Institute, Torrance, CA
Dysfunction of the skeletal muscles is an increasingly recognized causeof debility in a range of chronic disease. Only in the past few years has ske-letal muscle dysfunction been recognized in chronic obstructive pulmonarydisease [COPD]. Both strength training and endurance training seem to be
88 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

rational therapies; endurance training has been utilized with considerablesuccess. Studies have started to appear in which anabolic hormones havebeen administered to patients with COPD with the specific goal of enhan-cing muscle function.
Exercise Intolerance Is the Chief Complaint of Most Patients withCOPD. Dyspnea on exertion causes the COPD patient to assume an inactivelifestyle [4]. Chronic inactivity deconditions the muscles of ambulation,making activity even more unpleasant [2]. Until recently, considerationsfor therapy of exercise intolerance have focused on the pulmonary system.This is because patients with COPD have been presumed to be ventilatorylimited in their exercise tolerance. Ventilatory limitation is dictated bothby 1) greatly increased expiratory airflow resistance, leading to hyperinfla-tion and the inability to sustain relatively low levels of ventilation and 2)an increased ventilatory requirement for a given level of exercise becauseboth ventilation and gas exchange is inefficient. However, recent studieshave demonstrated that a substantial fraction of patients are primarily lim-ited by fatigue of the muscles of ambulation and not by ventilatory limitation[3, 26]. This has served to heighten the focus on interventions to amelioratemuscle dysfunction.
Evidence for Dysfunction of the Muscles of Ambulation in COPD. Astatement of the American Thoracic Society [8] summarized evidence thatmuscles of ambulation of COPD patients are dysfunctional. This evidenceincludes:
a. Muscle mass and strength are low. In comparison to matched controls,calf muscle cross-sectional area is reduced [1] and strength is low [1,19] in patients with COPD.
b. Muscle aerobic enzymes and capillarity is low. Vastus lateralis musclebiopsy specimens reveal, in comparison with age matched healthy con-trols, that COPD patients have 1) low levels of citrate synthase and hy-droxyacetyl CoA dehydrogenase, representative aerobic enzymes [23],as well as decreased mitochondrial electron transport chain enzymes[27], 2) decreased capillary density [32], 3) a decreased fraction of typeI fibers and 4) decreased fiber cross sectional area [32]. These changesare typical of muscles with a low aerobic capacity.
c. Early onset of lactic acidosis. Lactic acid is produced by exercising mus-cle when mitochondrial oxygen delivery becomes inadequate. In astudy of 33 COPD patients, the lactate threshold was, on average,58% of that seen in age matched healthy patients [2]. Others have con-firmed this finding [4]. This finding is consistent with magnetic reso-nance spectroscopy of COPD muscles, which shows that pH decreasesat abnormally low levels of exercise [24].
Poster Abstracts 89
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

d. VO2 kinetics are slow. When exercise begins, muscle O2 requirementsrise abruptly, yet VO2 measured at the mouth requires several minutesto reach a new steady state. This lag constitutes an oxygen deficit [31].Poor muscle function yields slow VO2 kinetics and a large oxygen def-icit. COPD patients have been shown to have markedly slow VO2
kinetics [10].e. Exercise intolerance persists after lung transplantation. After unilateral
or bilateral lung transplantation, exercise tolerance is no longer limitedby the achievable pulmonary ventilation. Nevertheless, several surgicalseries have reported maximum VO2 is in the range of 40–50%predicted [2]. These data suggest a source of exercise intolerancenot related to the pulmonary system, though they do not unequivocallyidentify the exercising muscles as the limiting factor [2].
f. Endurance exercise training improves exercise tolerance. Enduranceexercise training programs designed with a physiological rationaleincrease exercise tolerance in COPD patients [9, 10]. Evidence ofimproved muscle function has been obtained in patients with bothmoderate [9] and severe [10] disease.
Mechanisms of Muscle Dysfunction in COPD. The mechanisms produ-cing this dysfunction, and their relative importance, are subjects of activedebate.
a. Deconditioning. Acute deconditioning of healthy subjects yields de-creases in muscle capillary density, aerobic enzyme concentrationsand shifts from type IIa to type IIb fibers [12]; these changes resultin decreased muscle strength and endurance. These muscle character-istics are similar to those seen in COPD patients [23, 27, 32].
b. Malnutrition. A subset of COPD patients are appreciably underweight[29, 33]. However, even patients who have normal body weight havea high incidence of malnutrition and low muscle mass [16]. Low musclemass has been shown to correlate with decreased strength and exerciseendurance [28].
c. Skeletal muscle myopathy. A myopathic process may contribute to exer-cise tolerance in COPD, as it does in other chronic diseases (e.g.,chronic renal failure [5], congestive heart failure [8]). Many patientswith COPD are treated with corticosteroids; this has been shown toinduce myopathic changes [13, 14]. How long these myopathic changespersist after cessation of therapy is unclear. It seems possible thatchronic hypoxia [20], chronic hypercapnia [17] or the presence ofinflammatory mediators [25] contribute to muscle dysfunction.
d. Low circulating levels of anabolic hormones. Both growth hormoneand the androgenic steroids provide stimulation for muscle growth
90 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

and development. Accumulating data indicates that anabolic hormonelevels are low in COPD. Semple et al. found low testosterone levels inacutely ill, hospitalized COPD patients [30]. Our study of 87 ambula-tory COPD patients revealed substantially reduced IGF1 levels in bothmen and women [7]. In COPD men, testosterone levels are consider-ably below levels expected for age matched subjects [7]. The mechan-ism of these alterations is unclear, but it has been speculated thatchronic hypoxia [18, 30], corticosteroid therapy [15, 21] and chronicillness contribute to low testosterone levels.
Endurance Training Improves Muscle Function and Exercise Tolerancein COPD. High-intensity endurance exercise training program improvesmuscle function and exercise tolerance in COPD men and women [9, 10].Lower exercise intensities are much less effective [6, 9]. Further, quadricepsmuscle biopsies have confirmed that aerobic enzyme concentration andcapillary density are increased after exercise training [22]. Blood lactatelevels are lower at a given level of exercise [9] and VO2 kinetics are faster[10] in COPD patients after a rigorous endurance training program.
References
1. Bernard, S., P. LeBlanc, F. Whittom, G. Carrier, J. Jobin, R. Belleau, and F. Maltais. Peripheral muscleweakness in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med.158:629–634, 1998.
2. Casaburi, R. Deconditioning. In: Pulmonary Rehabilitation, Fishman, A.P., ed., Lung Biology in Healthand Disease Series, New York: Marcel Dekker, pp. 213–230, 1996.
3. Casaburi, R. Limitation to exercise tolerance in chronic obstructive pulmonary disease: Look to themuscles of ambulation. Am. J. Respir. Crit. Care Med. 168:409–410, 2003.
4. Casaburi, R. Exercise training in chronic obstructive lung disease. In: Principles and Practice of Pulmon-ary Rehabilitation, Casaburi, R. and T.L. Petty, eds., Philadelphia: Saunders, pp. 204–224, 1993.
5. Casaburi, R. Rehabilitative exercise training in chronic renal failure. In: Nutritional Management ofRenal Disease, Kopple, J.D. and S.G. Massry, eds., Baltimore: Williams and Wilkins, pp. 817–841, 1996.
6. Casaburi, R. Special considerations for exercise training in chronic lung disease. In: ACSM’s ResourceManual for Guidelines for Exercise Testing and Prescription, 3rd Edition, Baltimore: Williams and Wilkins,pp. 334–338, 1998.
7. Casaburi, R., S. Goren, and S. Bhasin. Substantial prevalence of low anabolic hormone levels inCOPD patients undergoing rehabilitation. Am. J. Respir. Crit. Care Med. 153:A128, 1996.
8. Casaburi, R., R. Gosselink, M. Decramer, R.P.N. Dekhuijzen, M. Fournier, M.I. Lewis, F. Maltais, D.A.Oelberg, M.B. Reid, J. Roca, A.M.W.J. Schols, G.C. Sieck, D.M. Systrom, P.D. Wagner, T.J. Williams,and E. Wouters. Statement of the ATS and ERS: Skeletal muscle dysfunction in chronic obstructivelung disease. Am. J. Respir. Crit. Care Med. 159:S1–S40, 1999.
9. Casaburi, R., A. Patessio, F. Loli, S. Zanaboni, C.F. Donner, and K. Wasserman. Reduction in exerciselactic acidosis and ventilation as a result of exercise training in obstructive lung disease. Am. Rev.Respir. Dis. 143:9–18, 1991.
10. Casaburi, R., J. Porszasz, M.R. Burns, E.R. Carithers, R.S.Y. Chang, and C.B. Cooper. Physiologic ben-efits of exercise training in rehabilitation of patients with severe chronic obstructive pulmonary dis-ease. Am. J. Respir. Crit. Care Med. 155:1541–1551, 1997.
11. Casaburi, R. and K. Wasserman. Exercise training in pulmonary rehabilitation (editorial). N. Engl.J. Med. 314:1509–1511, 1986.
Poster Abstracts 91
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

12. Coyle, E.F., W.H. Martin, D.R. Sinacore, M.J. Joyner, J.M. Hagberg, and J.O. Holloszy. Time course ofloss of adaptations after stopping prolonged intense endurance training. J. Appl. Physiol. 57:1857–1864, 1984.
13. Decramer, M., V. deBock, and R. Dom. Functional and histologic picture of steroid-induced myopa-thy in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 153:1958–1964, 1996.
14. Decramer, M., L.M. Lacquet, R. Fagard, and P. Rogiers. Corticosteroids contribute to muscle weak-ness in chronic airflow obstruction. Am. Rev. Respir. Dis. 150:11–16, 1994.
15. Doerr, P. and K.M. Pirke. Cortisol-induced suppression of plasma testosterone in normal adult males.J. Clin. Endocrinol. Metab. 43:622–629, 1976.
16. Engelen, M.P.K.J., A.M.W.J. Schols, W.C. Baken, G.J. Wesseling, and E.F.M. Wouters. Nutritionaldepletion in relation to respiratory and peripheral skeletal muscle function in outpatients withCOPD. Eur. Respir. J. 7:1793–1797, 1994.
17. Fiaccadori, E., S. Del Canale, P. Vitali, E. Coffrini, N. Ronda, and A. Guariglia. Skeletal muscle ener-getics, acid-base equilibrium and lactate metabolism in patients with severe hypercapnia and hypox-emia. Chest 92:883–887, 1987.
18. Gosney J.R. Atrophy of Leydig cells in the testes of men with longstanding chronic bronchitis andemphysema. Thorax 42:615–619, 1987.
19. Gosselink, R., T. Troosters, and M. Decramer. Peripheral muscle weakness contributes to exercise lim-itation in COPD. Am. J. Respir. Crit. Care Med. 153:976–980, 1996.
20. Howald, H., D. Pette, J.A. Simoneau, A. Uber, H. Hoppler, and P. Cerretelli. Effect of chronic hypoxiaon muscle enzyme activities. Int. J. Sports Med. 11:S10–S14, 1990.
21. MacAdams, M.R., R.H. White, and B.E. Chipps. Reduction of serum testosterone levels duringchronic glucocorticoid therapy. Annals. Intern. Med. 104:648–651, 1986.
22. Maltais, F., P. LeBlanc, C. Simard, J. Jobin, C. Berube, J. Bruneau, L. Carrier, and R. Belleau. Skeletalmuscle adaptation to endurance training in patients with chronic obstructive pulmonary disease. Am.Respir. Crit. Care Med. 154:442–447, 1996.
23. Maltais, F., A.A. Simard, C. Simard, J. Jobin, P. Desgagnes, and P. LeBlanc. Oxidative capacity of theskeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD.Am. J. Respir. Crit. Care Med. 153:288–293, 1996.
24. Payen, J.F., B. Wuyam, P. Levy, H. Reutenauer, P. Stieglitz, B. Paramelle, and J.F. Le Bas. Muscularmetabolism during oxygen supplementation in patients with chronic hypoxemia. Am. Rev. Respir.Dis. 147:592–598, 1993.
25. Reid, M.B. Reactive oxygen and nitric oxide in skeletal muscle. News Physiol. Sci. 11:114–119, 1996.26. Saey, D., R. Debigare, P. LeBlanc, M.J. Mador, C.H. Cote, J. Jobin, and F. Maltais. Contractile leg
fatigue after cycle exercise: a factor limiting exercise in patients with COPD. Am. J. Respir. Crit. CareMed. 168:425–430, 2003.
27. Sauleda, J., F. Garcıa-Palmer, R.J. Wiesner, S. Tarraga, I. Harting, P. Tomas, C. Gomez, C. Saus, A.Palou, and A.G.N. Agustı. Cytochrome oxidase activity and mitochondrial gene expression in skeletalmuscle of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med.157:1413–1417, 1998.
28. Schols, A.M.W.J., R. Mostert, P.B. Soeters, and E.F.M Wouters. Body composition and exercise perfor-mance in chronic obstructive pulmonary disease. Thorax 46:695–699, 1991.
29. Schols, A.M.W.J., J. Slangen, L. Volovics, and E.F.M Wouters. Weight loss is a reversible factor in theprognosis of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 157:1791–1797, 1998.
30. Semple, D’A.P., G.H. Beastall, W.S. Watson, and R. Hume. Serum testosterone depression associatedwith hypoxia in respiratory failure. Clin. Sci. 58:105–106, 1980.
31. Whipp, B.J., C. Seard, and K. Wasserman. Oxygen deficit-oxygen debt relationships and efficiency ofanaerobic work. J. Appl. Physiol. 28:452–456, 1970.
32. Whittom, F., J. Jobin, P.M. Simard, P. LeBlanc, C. Simard, S. Bernard, R. Belleau, and F. Maltais. His-tochemical and morphological characteristics of the vastus lateralis muscle in patients with chronicobstructive pulmonary disease. Med. Sci. Sports Exerc. 30:1467–1474, 1998.
33. Wilson, D.O., R.M. Rogers, E.C. Wright, and N.R. Anthonisen. Body weight in chronic obstructivepulmonary disease: The National Institutes of Health intermittent positive pressure breathing trial.Am. Rev. Respir. Dis. 139:1435–1438, 1989.
92 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

Quantitative Analysis of Computed Tomography Scans in ChronicObstructive Pulmonary Disease
H. O. CoxsonVancouver General Hospital, University of British Columbia, Vancouver, BC Canada
Chronic obstructive pulmonary disease [COPD] is currently the 12thleading cause of disability in the world and is predicted to be 5th by the year2020 [1]. In the United States alone, it has been estimated that the annualcost of morbidity and early mortality due to COPD is approximately 4.7 bil-lion dollars [2]. COPD is a complex genetic disorder in which environmen-tal factors interact with genetic susceptibility to cause disease. Tobaccosmoke is the most important environmental risk factor and in susceptibleindividuals it causes an exaggerated inflammatory response that ultimatelydestroys of the lung parenchyma (emphysema) and increases airway resis-tance by remodeling of the airway wall (Fig. 1).
The current definition of emphysema is expansion of the lung parench-yma beyond the normal range associated with destruction of the alveolarwalls [3]. Unfortunately, expansion beyond the normal range is a vaguedescription, because full expansion of the normal lung has not been welldefined by either morphology or CT. The advent of computed tomography(CT) has lead to numerous attempts to estimate emphysema in life. Thesestudies rely on the detection of relatively large radiolucent ‘‘holes’’ within
FIGURE 1 Tobacco smoke in susceptible individuals causes an exaggerated inflammatory response thatultimately destroys the lung parenchyma and increases airway resistance by remodeling the airway wall.
Poster Abstracts 93
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

the lung [4, 5]. The accuracy of CT in the diagnosis and assessment of sever-ity of emphysema has been well documented [6–10]. However, the correla-tion between a subjective assessment of emphysema and the pathologicseverity in various studies ranges been 0.4 to 0.9 and has been shown tobe less accurate than quantitative emphysema studies [6, 8, 11–15]. Threemain different approaches have been utilized for objective quantificationof emphysema on CT: (i) use of a threshold value below which emphysemais considered to be present [density mask or pixel index (PI)] [12, 16, 17](Fig. 2), (ii) assessment of the range of lung densities represented in a lungslice (histogram or percentile analysis) [18–22], and (iii) assessment of over-all lung density, often in combination with volumetric imaging [23–26].Pathologic estimates of macroscopic emphysema have been correlated withdensity mask and some percentile measurements [6, 16–19]. Mishima et al.have added an additional facet to the assessment of emphysema [27]. Bymeasuring the size of the clusters of low attenuation voxels, they were ableto quantify the size of the lesions and determined that as lesions become lar-ger, they become less in number. Coxson and colleagues proposed a methodto estimate the lung S/V based on an examination of the median value ofthe frequency distribution of the density (expressed as ml gas/g tissue) oflung voxels [17].
There are some major limitations to the above techniques. Firstly, thedensity mask or histogram techniques only measure the number of voxelsbeyond a cut-off value. These techniques do not allow discrimination
FIGURE 2 Example of pixel index graph. Index is CT of patient.
94 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

between low attenuation voxels caused by noise in the image reconstructionalgorithm and true low attenuation voxels. Secondly, both the histogram anddensity mask techniques only estimate the extent of large emphysematouslesions and do not adequately assess the effect of early or minor changes.Finally, it should be noted, that lung density measurements on CT can beaffected by a number of variables including patient size, depth of inspira-tion, the type of CT scanner used, collimation and the reconstruction algo-rithm. Therefore, the analysis requires careful attention to technique.
Objective Measurements of Airway Dimensions
Unlike the parenchyma, the airways have proven very difficult to mea-sure. The first attempts at measuring airways objectively involved manuallytracing the airway with a digitizer on the printed image [28–31]. Obviouslimitations of this technique are that it is very sensitive to the display para-meters of the printed image and is extremely cumbersome, time consumingand associated with considerable intra and inter-observer variability.
Several groups of investigators have assessed the use of automated imageanalysis for measurement of airway dimensions. McNitt-Gray and co-workersreported that the airway lumen area could be accurately measured using athreshold cut-off of 7500 HU [32]. The lumen area is calculated by count-ing the number of voxels within the 7500 HU boundary. King et al. used anexcised, fixed pig lung and found that a threshold cut-off of 7577 HUproduced the least error in lumen measurements [33].
A popular method to measure airway wall thickness is the full width athalf maximum method (‘‘half-max’’) [34] (Fig. 3). This approach casts raysfrom the airway centroid through the airway wall and measures the x-rayattenuation curve along the ray as it passes from the airway lumen throughthe wall and into the lung parenchyma. This technique assumes that theimage gray level at the true airway wall will be halfway between the mini-mum and maximum gray levels. However, Reinhardt pointed out that thescanning process introduces blurring and partial-volume effects and may,therefore, not be uniform across structures of different sizes [35]. Theyshowed that using this technique the airway lumen will be underestimatedand the airway wall thickness overestimated. For this reason they developeda technique known as ‘‘maximum-likelihood method’’ to estimate the wallthickness [35]. Airway measurements are also influenced by the airwayangle relative to the plane of section, airway size and beam collimation[30, 33]. Optimal measurements require use of sections 2 mm or less inthickness and are currently limited to airways 2 mm or more in diameter[34]. The majority of studies assessing airway measurements have limitedthe analysis to airways coursing perpendicular to the plane of the CTsection, i.e., airways cut in cross section. These airways appear round and
Poster Abstracts 95
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

have even wall thickness and are therefore relatively easy to measure. Air-ways coursing obliquely are oval in shape. However, because of variousfactors of image reconstruction many airways that are oblique to the planeof section may still appear to be round. Therefore, assessment of airwaysrequires correction for the angle of orientation [30, 33]. King and cowork-ers assessed the angle of orientation of the airways by determining the posi-tion of the centroid in three adjacent CT slices [33].
Nakano and coworkers evaluated the apical right upper lobe bronchusof 114 smokers using the half-max method [36]. They chose this airwaybecause it is usually cut in cross section on CT and is consistently and reliablyidentified on CT. These authors showed that the thickening in this large air-way correlated with abnormalities in lung function. The correlation withFEV1 % Predicted, FVC % Predicted and RV/TLC observed in this studysuggests that airway wall thickening and lumen narrowing of large airwaysresults in airflow obstruction. Furthermore, a multiple regression analysissuggested that, for a given FEV1, subjects with more extensive emphysemahad less airway wall thickening than those with less extensive emphysema.However, all symptomatic smokers had thicker walls than asymptomatic smo-kers [36].
Airway wall dimensions are very important measurements to obtain,however further studies are required to determine the potential role ofCT in the evaluation of the severity of airway wall inflammation and airwayobstruction in patients with COPD.
In summary, CT scans provide quantitative data that can be used toassess both the lung parenchyma and the airway wall dimensions in patientswith COPD. These studies are valuable for the study of the pathogenesis ofdisease and possible pharmacologic and surgical intervention.
FIGURE 3 Computer generated image of half maximum method for measuring airway wall thickness.
96 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

References
1. Murray, C. J. L. and A. D. Lopez. 1996. Evidence-based health policy—lessons from the Global Bur-den of Disease Study. Science 274:740–743.
2. Rossi, A. and M. Confalonieri. 2000. Burden of chronic obstructive pulmonary disease. Lancet356[Suppl]:s56.
3. American Thoracic Society. 1995. Standards for the diagnosis and care of patients with chronic ob-structive pulmonary disease. Am J Respir Crit Care Med 152:S77–S120.
4. Hruban, R. H., M. A. Meziane, E. A. Zerhouni, N. F. Khouri, E. K. Fishman, P. S. Wheeler, S. Dumler,and G. M. Hutchins. 1987. High resolution computed tomography of inflation-fixed lungs: Patholo-gic-radiologic correlation of centrilobular emphysema. Am Rev Respir Dis 136:935–940.
5. Webb, W. R., M. G. Stein, W. E. Finkbeiner, J. G. Im, D. Lynch, and G. Gamsu. 1988. Normal anddiseased isolated lungs: high-resolution CT. Radiology 166:81–87.
6. Miller, R. R., N. L. Muller, S. Vedal, N. J. Morrison, and C. A. Staples. 1989. Limitations of computedtomography in the assessment of emphysema. Am Rev Respir Dis 139:980–983.
7. Thurlbeck, W. M., and N. L. Muller. 1994. Emphysema: definition, imaging, and quantification. AJRAm J Roentgenol 163:1017–1025.
8. Kuwano, K., K. Matsuba, T. Ikeda, J. Murakami, A. Araki, H. Nishitani, T. Ishida, K. Yasumoto, andN. Shigematsu. 1997. The diagnosis of mild emphysema. Correlation of computed tomographyand pathology scores. Am Rev Respir Dis 141:169–178.
9. Gevenois, P. A., J. Zanen, V. de Maertelaer, P. De Vuyst, P. Dumortier, and J.-C. Yernault. 1995. Macro-scopic assessment of pulmonary emphysema by image analysis. J Clin Pathol 48:318–322.
10. Bankier, A. A., V. De Maertelaer, C. Keyzer, and P. A. Gevenois. 1999. Pulmonary emphysema: subjec-tive visual grading versus objective quantification with macroscopic morphometry and thin-sectionCT densitometry. Radiology 211(3):851–858.
11. Spouge, D., J. R. Mayo, W. Cardoso, and N. L. Muller. 1993. Panacinar emphysema: CT and patho-logic findings. J Comput Assist Tomogr 17:710–713.
12. Gevenois, P. A., V. de Maertelaer, P. De Vuyst, J. Zanen, and J. C. Yernault. 1995. Comparison of com-puted density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med152:653–657.
13. Remy-Jardin, M., J. Remy, B. Gosselin, M. C. Copin, A. Wurtz, and A. Duhamel. 1996. Sliding thinslab, minimum intensity projection technique in the diagnosis of emphysema: Histopathologic-CTcorrelation. Radiology 200:665–671.
14. Bankier, A. A., D. Fleischmann, R. Mallek, A. Windisch, F. W. Winkelbauer, M. Kontrus, L. Havelec,C. J. Herold, and P. Hubsch. 1996. Bronchial wall thickness: Appropriate window settings for thin-section CT and radiologic-anatomic correlation. Radiology 199(3):831–836.
15. Uppaluri, R., T. Mitsa, M. Sonka, E. A. Hoffman, and G. McLennan. 1997. Quantification of pulmon-ary emphysema from lung computed tomography images. Am J Respir Crit Care Med 156(1):248–254.
16. Muller, N. L., C. A. Staples, R. R. Miller, and R. T. Abboud. 1988. ‘‘Density mask.’’ An objective meth-od to quantitate emphysema using computed tomography. Chest 94:782–787.
17. Coxson, H. O., R. M. Rogers, K. P. Whittall, Y. D’Yachkova, P. D. Pare, F. C. Sciurba, and J. C. Hogg.1999. A quantification of the lung surface area in emphysema using computed tomography. AmJ Respir Crit Care Med 159(3):851–856.
18. Hayhurst, M. D., D. C. Flenley, A. McLean, A. J. A. Wightman, W. MacNee, D. Wright, D. Lamb, andJ. Best. 1984. Diagnosis of pulmonary emphysema by computerized tomography. Lancet 2:320–322.
19. Gould, G. A., W. MacNee, A. McLean, P. M. Warren, A. Redpath, J. J. Best, and D. Lamb. 1988. CTmeasurements of lung density in life can quantitate distal airspace enlargement—an essential defin-ing feature of human emphysema. Am Rev Respir Dis 137:380–392.
20. Bae, K. T., R. M. Slone, D. S. Gierada, R. D. Yusen, and J. D. Cooper. 1997. Patients with emphysema:quantitative CT analysis before and after lung volume reduction surgery. Work in progress. Radiology203(3):705–14.
21. Dirksen, A., M. Friis, K. P. Olesen, L. T. Skovgaard, and K. Sorensen. 1997. Progress of emphysema insevere a1-antitrypsin deficiency as assessed by annual CT. Acta Radiologica 38:1–7.
22. Dirksen, A. 1999. A randomized clinical trial of a-1 antitrypsin augmentation therapy. Am J Respir CritCare Med 160:1468–1472.
Poster Abstracts 97
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

23. Zagers, H., H. A. Vrooman, N. J. Aarts, J. Stolk, L. J. Schultze Kool, J. H. Dijkman, A. E. Van Voorthui-sen, and J. H. Reiber. 1996. Assessment of the progression of emphysema by quantitative analysis ofspirometrically gated computed tomography images. Invest Radiol 31(12):761–767.
24. Brown, M. S., M. F. McNitt-Gray, J. G. Goldin, L. E. Greaser, U. M. Hayward, J. W. Sayre, M. K. Arid,and D. R. Aberle. 1999. Automated measurement of single and total lung volume from CT. J ComputAssist Tomogr 23(4):632–640.
25. Kauczor, H.-U., C. P. Heussel, B. Fischer, R. Klamm, P. Mildenberger, and M. Thelen. 1998. Assess-ment of lung volumes using helical CT at inspiration and expiration: Comparison with pulmonaryfunction tests. AJR Am J Roentgenol 171(4):1091–1095.
26. Mergo, P. J., W. F. Williams, R. Gonzalez-Rothi, R. Gibson, P. R. Ros, E. V. Staab, and T. Helmberger.1998. Three-dimensional volumetric assessment of abnormally low attenuation of the lung from rou-tine helical CT: inspiratory and expiratory quantification. AJR Am J Roetgenol 170(5):1355–1360.
27. Mishima, M., T. Hirai, H. Itoh, Y. Nakano, H. Sakai, S. Muro, K. Nishimura, Y. Oku, K. Chin, M. Ohi,T. Nakamura, J. H. T. Bates, A. M. Alencar, and B. Suki. 1999. Complexity of terminal airspace geo-metry assessed by lung computed tomography in normal subjects and patients with chronic obstruc-tive pulmonary disease. Proc Natl Acad Science USA 96:8829–8834.
28. Seneterre, E., F. Paganin, J. M. Bruel, F. B. Michel, and J. Bousquet. 1994. Measurement of the inter-nal size of bronchi using high resolution computed tomography (HRCT). Eur Respir J 7:596–600.
29. Okazawa, M., N. L. Muller, A. E. McNamara, S. Child, L. Verburgt, and P. D. Pare. 1996. Human air-way narrowing measured using high resolution computed tomography. Am J Respir Crit Care Med154:1557–1562.
30. Webb, W. R., G. Gamsu, S. D. Wall, C. E. Cann, and E. Proctor. 1984. CT of a bronchial phantom:Factors affecting appearance and size measurements. Invest Radiol 19:394–398.
31. McNamara, A. E., N. L. Muller, M. Okazawa, J. Arntorp, B. R. Wiggs, and P. D. Pare. 1992. Airwaynarrowing in excised canine lung measured by high-resolution computed tomography. J Appl Physiol73:307–316.
32. McNitt-Gray, M. F., J. G. Goldin, T. D. Johnson, D. P. Tashkin, and D. R. Aberle. 1997. Developmentand testing of image-processing methods for the quantitative assessment of airway hyperresponsive-ness from high-resolution CT images. J Comput Assist Tomogr 21(6):939–947.
33. King, G. G., N. L. Muller, K. P. Whittall, Q. S. Xiang, and P. D. Pare. 2000. An analysis algorithm formeasuring airway lumen and wall areas from high-resolution computed tomographic data. Am JRespir Crit Care Med 161:574–580.
34. Nakano, Y., K. P. Whittall, S. E. Kalloger, H. O. Coxson, and P. D. Pare. 2002. Development andvalidation of human airway analysis algorithm using multidetector row CT. Proc SPIE 4683:460–469.
35. Reinhardt, J. M., N. D. D’Souza, and E. A. Hoffman. 1997. Accurate measurement of intrathoracicairways. IEEE Trans Med Imaging 16(6):820–827.
36. Nakano, Y., S. Muro, H. Sakai, T. Hirai, K. Chin, M. Tsukino, K. Nishimura, H. Itoh, P. D. Pare, J. C.Hogg, and M. Mishima. 2000. Computed tomographic measurements of airway dimensions and em-physema in smokers. Correlation with lung function. Am J Respir Crit Care Med 162:1102–1108.
Exercise Limitation in COPD: Ventilatory Factors
D. E. O’DonnellDepartment of Medicine, Queen’s University, Kingston, Ontario, Canada
Exercise intolerance is very common in patients with advanced COPDand contributes importantly to an impoverished quality of life. Recentresearch has confirmed the complex nature of exercise limitation and hasshown that the predominant contributory factors can vary from patient topatient. Recognized exercise limiting factors include: (1) incapacitating
98 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

exertional symptoms, (2) derangements of ventilatory mechanics and mus-cle function, (3) gas exchange and metabolic abnormalities, (4) peripheralmuscle dysfunction, (5) impaired cardiac function, and (6) any combinationof the above [1]. Abnormalities of ventilatory mechanics are fundamental toexercise limitation in COPD and influence (directly or indirectly) all of theabove listed factors in a complex integrative manner. This review will focuson the nature of the altered dynamic mechanics during exercise in COPDand show that this can be therapeutically manipulated for the patient’sbenefit.
Expiratory flow limitation (EFL) is the pathophysiological hallmark ofCOPD and occurs because of a combination of airway luminal factors(i.e., mucosal inflammation, edema, fibrosis), extraluminal factors (i.e.,reduced tethering, compression by adjacent overdistended alveoli) and var-iation in bronchomotor tone [2]. In emphysema, reduced lung recoildecreases the driving pressure for expiratory flow, further compoundingEFL. Under conditions of EFL, expiratory flow is effort-independent andduring quiet breathing lung emptying becomes critically dependent onexpiratory time [3]. In COPD, the time constant of the respiratory systemexceeds the available expiratory time during tidal breathing and alveolargas retention (or air trapping) is inevitable. Therefore, in contrast to health,end-expiratory lung volume (EELV) is a continuous dynamic variable that isgenerally higher than the predicted relaxation volume of the respiratory sys-tem (i.e., functional residual capacity).
Dynamic Lung Hyperinflation
When ventilation increases, either voluntarily or during exercise, EELVincreases above its resting value because the interval between successivebreaths is now even shorter and lung emptying is further compromised(Figure 1). This acute-on-chronic hyperinflation is termed dynamic hyper-inflation (DH). The extent of DH during exercise is variable and dependson (1) the level of resting hyperinflation (inversely related), (2) the extentof EFL, and (3) the prevailing ventilatory demand. In 105 patients with mod-erate to severe COPD, the change in inspiratory capacity (IC) (whichreflects the change in EELV as TLC remains constant) was 0.37 L, on aver-age, with considerable variation in the range [4]. Therefore, at peak exer-cise, IC was reduced by an average of 18% of its already reduced restingvalue. Patients with an emphysematous clinical profile have faster rates ofDH earlier in exercise than patients with a chronic bronchitis profile(matched for FEV1.0 but with a preserved diffusion capacity for carbon mon-oxide). The rest-to-peak change in EELV was similar in chronic bronchitisand emphysema, but in the former group, occurred later in exercise whenventilation increased to higher levels [4]. In emphysematous patients, DH is
Poster Abstracts 99
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

amplified by greater EFL and higher ventilatory demands because of greaterventilation-perfusion abnormalities (high physiological deadspace). Thesepatients had greater exertional dyspnea and exercise intolerance [4].
In flow-limited patients, the resting IC (% predicted) correlates well withpeak symptom-limited oxygen uptake (VO2) [5]. The IC is often surprisinglywell preserved at rest, even in patients with advanced emphysema (TLC andFRC increase together) [6]. However, as the resting IC diminishes (<70%predicted), ventilatory limitation to exercise becomes more likely [4, 5].The IC represents the true operating limits for tidal volume (VT) expansionduring exercise. The lower the IC, the lower the peak VT achieved (Figure 2)and, consequently, the earlier the ventilatory limitation to exercise in theface of the increasing central drive to breathe. Progressive reduction of ICduring exercise means that the VT is positioned closer to TLC, where thereis increased elastic and inspiratory threshold loading as well as weakening of
FIGURE 1 Comparative pulmonary function graphs depicting averaged EELV and IC for normal versusCOPD patients.
FIGURE 2 Graphic presentation of IC and VT in tested COPD population.
100 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

the inspiratory muscles [2]. In hyperinflated patients, reliance on increasedbreathing frequency to increase ventilation rebounds to cause further DH ina vicious cycle. In patients with advanced COPD and severe gas exchangeabnormalities, the restrictive mechanical consequences of DH (reducedVT) result in overt respiratory insufficiency and exercise hypercapnia [7].
Dynamic Hyperinflation and Dyspnea in COPD
The intensity of dyspnea during exercise rises precipitously as theinspiratory reserve volume (IRV) declines relatively rapidly (because of airtrapping) to a critically low value of approximately 0.5 L [8]. Dyspnea inten-sity correlates well with the reduced IC and IRV during exercise. DH, byloading and weakening the inspiratory muscles, causes heightened inspira-tory effort at any given ventilation. This increased effort is reflected by ahigh ratio of tidal esophageal pressure to the maximal possible pressure thatcan be generated at that volume [10]. In the exercising COPD patient thereis, therefore, a disparity or dissociation between the central drive to breathe(which is invariably amplified) and the mechanical response of the system,which is greatly constrained due to DH [9]. This neuromechanical dissocia-tion may form the basis for the perception of respiratory discomfort, parti-cularly the discrete qualitative descriptor of ‘‘unsatisfied inspiration’’ thatcharacterizes COPD.
Deflating the Lungs in COPD
Indirect evidence for the importance of DH in exercise limitation anddyspnea causation has come from a number of interventional studies[11–13]. Thus, alleviation of exertional dyspnea and improved exerciseendurance as a result of bronchodilators has been shown to be linked toincreased resting and dynamic IC and IRV [11, 12]. Bronchodilator-inducedimprovements in operating lung volumes reflect enhanced small airwayfunction (and lung emptying) and can occur in the absence of any changein FEV1.0. Similarly, improvements in dyspnea after lung volume reductionsurgery has been shown to correlate with reduced EELV and enhancedneuromechanical coupling of the diaphragm [13].
Interventions that reduce ventilatory demand, such as oxygen therapy(which alters chemoreceptor activity), reduce DH in patients with EFL[14–16]. IRV increases during exercise in a dose-response relation to incre-mental oxygen, even in normoxic COPD patients [16]. These mechanicalimprovements and the attendant improvement in dyspnea represent oneimportant contributor to improved exercise performance during oxygentherapy [14, 15].
Poster Abstracts 101
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

In summary, lung overinflation and the consequent restrictive mechan-ical constraints lead to early ventilatory limitation of exercise in patients withadvanced COPD. Improvement in exercise performance following pharma-cological (including oxygen therapy) and surgical lung volume reductionresults, in part, from a delay in this critical mechanical limitation. The rateand extent of DH can be reliably measured in COPD and such measure-ments should be considered as useful physiological outcomes for the evalua-tion of the clinical efficacy of various pharmacological interventions.
References
1. O’Donnell DE. Exercise limitation and clinical exercise testing in chronic obstructive lung disease.In: Clinical Exercise Testing: Progress in Respiratory Research. Eds. J. Zebellos, I. Weisman. KargerSeries, 2002; 32:138–158.
2. Pride NB, Macklem PT. Lung mechanics in disease. In: AP Fishman, ed. Handbook of Physiology.Section 3, Vol III, Part 2: The Respiratory System. Bethesda, MD: American Physiological Society1986; 659–692.
3. Hyatt RE. Expiratory flow limitation. J Appl Physiol 1983; 55:1–8.4. O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in COPD. Am
J Respir Crit Care Med 2001; 164:770–777.5. Diaz O, Villafranco C, Ghezzo H, Borzone G, Leiva A, Milic-Emili J, Lisboa C. Exercise tolerance in
COPD patients with and without tidal expiratory flow limitation at rest. Eur Respir J 2000; 16:269–275.6. Newton M, O’Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large po-
pulation of patients with severe hyperinflation. Chest 2002; 121(4):1042–1050.7. O’Donnell DE, D’Arsigny C, Fitzpatrick M, Webb KA. Exercise hypercapnia in advanced COPD: The
role of lung hyperinflation. (Accompanying editorial by J. Dempsey, pp 634–635). Am J Respir CritCare Med 2002; 166:663–668.
8. O’Donnell DE, Webb KA. Exertional breathlessness in patients with CAL: The role of lung hyperin-flation. Am Rev Respir Dis 1993; 48(5):1351–1357.
9. O’Donnell DE, Chau LL, Bertley J, Webb KA. Qualitative aspects of exertional breathlessness in CAL:Pathophysiological mechanisms. Am J Respir Crit Care Med 1997; 155:109–115.
10. Killian KJ, Gandevia SC, Summers E, Campbell EJM. Effect of increased lung volume on perceptionof breathlessness, effort and tension. J Appl Physiol 1984; 57:686–691.
11. Belman MJ, Botnick WC, Shin JW. Inhaled bronchodilators reduce dynamic hyperinflation duringexercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996;153:967–975.
12. O’Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation and enduranceduring exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;158:1557–1565.
13. Martinez FJ, Montes de Oca M, Whyte RI, Stetz J, Gay SE, Celli BR. Lung-volume reduction improvesdyspnea, dynamic hyperinflation and respiratory muscle function. Am J Respir Crit Care Med 1997;155:1984–1990.
14. O’Donnell DE, D’Arsigny C, Webb KA. Effects of hyperoxia on ventilatory limitation during exercisein advanced COPD. Am J Respir Crit Care Med 2001; 163:892–898.
15. O’Donnell DE, Bain DJ, Webb KA. Factors contributing to relief of exertional breathlessness duringhyperoxia in chronic airflow limitation. Am J Respir Crit Care Med 1997; 166:530–535.
16. Somfay A, Porszasz J, Lee SM, Casaburi R. Dose-response effect of oxygen on hyperinflation andexercise endurance in nonhypoxaemic COPD patients. Eur Respir J 2001; 18:77–84.
102 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

Lower Respiratory Tract Inflammation in COPD
S. RennardUniversity of Nebraska Medical Center, Omaha, NE
Inflammation has been long recognized as a characteristic feature ofchronic obstructive pulmonary disease (COPD). Its importance in thepathogenesis of the condition has recently been highlighted by the inclusionof inflammation in the definition of COPD adopted in the recent GOLDGuidelines. Renewed interest in inflammation in COPD stems from the con-cept that treating inflammation is a potential means to alter the relentlesslyprogressive natural history of the disorder. This has led to novel applicationsof older methods for assessing lower respiratory tract inflammation as wellas the development of newer methods that hold great promise for non-inva-sive assessment of patients.
COPD is heterogeneous, and the inflammatory response of the lowerrespiratory tract can differ from patient to patient. In addition, inflamma-tory cell accumulation and activation can differ in various locations in thelower respiratory tract. Neutrophils, for example, are present in increasednumbers within intraluminal space and can be sampled through bronchoal-veolar lavage. Their numbers, however are not increased in the airway wallin patients with mild disease, perhaps because their transit through the air-way is relatively rapid. With worsening disease, however, increased numbersof neutrophils accumulate within glands, within airway walls and withinalveoli. The prominent role for neutrophils and their mediators in thepathogenesis of COPD is supported not only by observations in patients,but also by animal studies. Macrophages also accumulate within the airwaysof both smokers and individuals with COPD. Animal studies have suggesteda key role for macrophage-derived mediators, including metalloproteases inthe pathogenesis of COPD. Other inflammatory cells likely also play a rolein COPD. Lymphocytes are present, particularly CD8þ T cells, the accumu-lation of which correlates with disease severity. Taken together, these obser-vations support the concept that COPD is characterized by a heterogeneousinflammatory process that evolves as disease progresses and may differ frompatient to patient. A number of key questions remain unanswered. Mostimportant is whether treatments targeting inflammatory processes willimprove the natural history of COPD. Corollary questions are: Why doesthe inflammation caused by cigarette smoke lead to more severe diseasein some individuals? Why does inflammation seem to persist despite smok-ing cessation in some patients with COPD? Recent methodologic advancespromise to help address these issues.
Poster Abstracts 103
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

The ‘‘classical’’ method for assessing lower respiratory tract inflamma-tion is histology, which remains the gold standard. The relative difficultyin obtaining large tissue specimens required early analyses to be performedeither at autopsy or on tissues removed during surgical procedures, mostoften for cancer. Biopsy through the flexible fiberoptic bronchoscope,widely used for diagnostic studies, has been used recently to obtain tissuefor research purposes. Published results to date have been limited to sam-ples of proximal airways, although the method can sample alveolar struc-tures as well. This method can not only characterize and quantify lowerrespiratory tract inflammation but, in at least one recent trial, has been usedto demonstrate an improvement in lymphocyte populations following a ther-apeutic intervention. Other methods of sampling the lower respiratory tractthrough the bronchoscope include bronchoalveolar lavage, which can sam-ple the intraluminal contents of both the airways and the alveoli, and endo-bronchial brushings, which can obtain both proximal and distal airwayepithelial cells. Ability to access the lower respiratory tract provides thepotential not only for routine histological and immunohistochemical stu-dies, but also provides the possibility for genomic and proteomic analyses.
Less invasive methods than bronchoscopy are also being explored. Thetechnique of induced sputum can sample the lower respiratory tract ofapproximately two-thirds of individuals. This method samples intraluminalcontents, and patients with COPD are characterized by increased numbersof neutrophils and increased inflammatory cytokines compared to controlsmokers and non-smokers. Importantly, the inflammatory features sampledby induced sputum in COPD differ from asthma. Markers of inflammationare also present in exhaled breath condensate. Because of its almost comple-tely non-invasive nature, this method holds great promise for repeated sam-pling in individuals and for sampling in large numbers of subjects. Patientswith COPD have been reported to have increased levels of hydrogen perox-ide and increased levels of lipid peroxidation products. Increased nitricoxide in the exhaled breath is characteristic of active disease in asthma.The ease of measurement has made it an attractive parameter to monitortreatment in both research and clinical settings.
The ability to assess lower respiratory tract inflammation in COPD holdsgreat promise. Not only will greater understanding of the pathogenesis ofCOPD likely result, but it seems likely that assessment of lower respiratorytract inflammation will become a key feature in the evaluation of noveltherapies and likely that assessment of lower respiratory tract inflammationwill become a routine clinical tool.
104 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

Proliferating Epithelial Cells Are not Removed During Recoveryof Metaplastic Changes Induced by Inflammation
J. F. Harris1, J. Harkema2 and Y. Tesfaigzi1,1Lovelace Respiratory Research Institute, Albuquerque, NM; 2College of Veterinary Medicine,Michigan State University, East Lansing, MI
All people are subjected to inflammatory responses that cause mucouscell metaplasia (MCM) and increased mucous secretions following expo-sures to environmental toxins, allergens, and/or viral and bacterial infec-tions. However, only a few people develop chronic airway diseases, such asasthma and chronic bronchitis, with sustained MCM and mucous secretionsthat cause airway dysfunction. It is crucial to understand the normal resolu-tion of MCM in order to develop strategies to reduce mucus-producing cells.Our previous studies demonstrated the proliferation of epithelial cells andan increase in the number of total epithelial cells following LPS-inducedinflammation in the rat lung and that this increase mainly consists of meta-plastic mucous cells. The present study was designed to examine thechanges in cell numbers in the epithelium and whether proliferated cellsare removed during the recovery of the epithelium from this inflammatoryprocess. F344/N rats were intratracheally instilled with 1000mg LPS andinjected with BrdU to label all proliferating cells, then sacrificed at timepoints ranging from 2 to 40 days post-LPS instillation. Similar to our previousstudy, the number of total epithelial cells increased from approximately 115to 175 per millimeter basal lamina (mmBL), with the mucous cells (MCs)increasing from <2 to 30 per mmBL at Day 3 post instillation. The numberof epithelial cells decreased by approximately 40 cells per mmBL, or by 25percent of the total, from 3 to 8 days post LPS instillation. Additionally,the number of mucus-storing cells decreased by 32 cells per mmBL, or by24 percent of the total MCs. BrdU positive nuclei, indicating cells that pro-liferated in response to the inflammation, were present at Day 2 post-instil-lation, and these numbers remained unchanged at approximately 16 cellsper mmBL throughout the resolution process. Because 50% of the MCswere BrdU positive, we assume that only 50% of the mucous cells could havebeen removed as part of the resolution. To study our hypothesis that apop-totic regulators are involved in the removal of these cells, we determined thenumber of mucous cells that were immunopositive for Bcl-2. The number ofBcl-2 positive MCs increased from <1 per mmBL at 2 days to 10 cells permmBL at 3 days post LPS instillation. During the following 5 days, the num-ber of Bcl-2 positive MCs was reduced to <1 per mmBL. Because 50% of theBcl-2 positive MCs were BrdU positive, only 50% of the Bcl-2 positive cellscould have been removed from the epithelium during the resolution
Poster Abstracts 105
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

process. Together, these observations suggest that the proliferating cells arenot targeted for removal during the resolution of metaplastic changes.Furthermore, it appears that only 50% of all MCs and 50% of the Bcl-2 posi-tive MCs could have been removed because the other 50% were BrdU posi-tive (and could not have been removed as all BrdU-positive cells remainedafter resolution). Future studies will focus on identifying the markers forcells that are removed during the resolution process. These studies may helpto elucidate the mechanisms sustaining metaplastic changes in chronic air-way diseases and identify novel targets that may help to reduce the numbersof mucus-producing cells. Supported by NIH ES09237.
COPD, Smokers and Non-Smokers Have Differential BALF ProteinProfiles by SELDI-TOF
B. Crowder1, R. Henderson1, S. Boggs2, and S. Rennard2
1Lovelace Respiratory Research Institute, Albuquerque, NM; 2University of Nebraska MedicalCenter, Omaha, NB
The use of surface enhanced laser desorption ionization time of flight(SELDI-TOF) mass spectrometry has become a useful diagnostic tool indetermining protein expression profiles of clinically relevant samples.SELDI-TOF utilizes ProteinChip Arrays, which carry functional groups thatserve to capture subsets of proteins from complex biological samples; suchas serum, plasma, bronchoalveolar lavage fluid, and urine. Once the pro-teins are bound to the surface, the chips may be washed to remove anyunbound proteins, salts and detergents. The proteins and peptides are thendetected by time-of-flight mass spectrometry and analyzed by ProteinChipSoftware 3.1.1.
Analysis of human bronchoalveolar lavage fluid (BALF) has revealed sev-eral protein/peptide biomarkers (p< 0.05) in the mass range of 2–10 kDathat delineate patients with chronic obstructive pulmonary disease (COPD),smokers who have not presented with the disease and non-smokers. Smokersin general have intermediary protein/peptide expression profiles comparedto COPD and non-smokers. We are currently undertaking the identificationof these biomarkers by several proteomic techniques.
106 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

The Effect of Cigarette Smoke on Human Lung Epithelial Permeability
D. Olivera1,2, D. Olivera2, C. Beenhouwer2, L. Herrera2, S. Boggs2, andC. Knall2
1University of New Mexico Health Sciences Center, College of Pharmacy, Albuquerque, NM;2Lovelace Respiratory Research Institute, Albuquerque, NM
Cigarette smoking is the main contributing factor in the development ofChronic Obstructive Pulmonary Disease (COPD), a disease characterized bya progressive destruction of lung tissue and loss of respiratory function.Increased permeability of the airway epithelium is a hallmark of this disease,and cells from the lungs of COPD patients show increased permeabilityupon cigarette smoke exposure compared to those from healthy smokers.Reversible changes in airway permeability are the result of tight junctionmodulation, rather than cell death, overt smoke toxicity, or protein degrada-tion. Research in non-respiratory systems has indicated that tight junctionfunction is regulated by the phosphorylation of occluding and ZO-1, tightjunction proteins, which alters their ability to participate in functional junc-tions. The mechanism controlling changes in airway epithelial permeabilityupon cigarette smoke exposure is unknown, however. We have begun toaddress this question using an air/liquid interface exposure system and clas-sic proteomics techniques. The Cultex apparatus allows cells to be exposedto freshly generated, whole cigarette smoke at an air/liquid interface. Todate, this represents the most physiologically relevant in-vitro exposure sys-tem available. We have used fluorescently-labeled albumin combined withconfocal microscopy to assess loss of barrier function to macromolecules.Analysis has revealed that macromolecular permeability manifests as focalaccumulations of the albumin in the basolateral spaces. Our evidence indi-cates that the size of these events increases in a dose-dependent mannerwith cigarette smoke exposure, and is the primary means by which increasedprotein flux across the epithelium occurs, as opposed to increasing eventfrequency. Interestingly, this regulated permeability to macromolecules ismost pronounced well after trans-epithelial electrical resistance (TER) isrestored. This indicates that permeability to ion flux and protein flux aretwo independently regulated processes, both of which are impacted by main-stream cigarette smoke. Forthcoming experiments will investigate the rolesof specific regulators of tight junction protein phosphorylation (PKC,PI3K, MEK, Src, ROCK, PTP1, and PP2A) in smoke-induced permeabilitychanges. This research is supported by funding from the Lovelace Respira-tory Research Institute and from the Johnson & Johnson Corporation.
Poster Abstracts 107
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

P Screening for Lung Cancer with Low-Dose Spiral ComputedTomography
J. R. Jett, D. E. Midthun, T. E. Hartman, and S. J. SwensenMayo Clinic, Rochester, MN
Lung cancer is the number one cancer killer in North America. Cur-rently, screening for lung cancer is not recommended. Therefore, patientswill not be diagnosed until they present with symptomatic disease, whichis usually advanced stage disease. Previous trials of screening with chestroentgenograms and sputum cytology have failed to show a decrease in lungcancer mortality. Recent reports of screening with low dose spiral CT havedetected lung cancers at a smaller size, average of 1.5 cm, than those usuallydetected by chest radiographs (mean of 3.0 cm). Spiral CT has been shownto detect between 58% and 85% of non-small cell lung cancers while theyare stage IA and this compares favorably to the current medical practicewhere only 15% are detected as localized disease (SEER data). This articlesummarizes the spiral CT screening data and emphasized the results ofthe Mayo Clinic spiral CT screening trial.
In 2003 there will be approximately 172,000 cases of lung cancer in theUSA. The overall five-year survival is 15% [1]. Lung cancer alone accountsfor more cancer deaths than the next four most common cancer causes ofdeath combined (Table 1). Currently, 47% of all lung cancers occur inwomen and more women die of lung cancer than breast cancer in America(68,800 versus 39,800). What is striking from Table 1 is the disparity in thefive-year survival for lung cancer compared to the other most common can-cer causes of death. Of these common cancer killers, lung cancer and pan-creatic cancer are the only ones for which screening is not recommended.We also know that symptomatic lung cancer is usually an advanced cancer,stage IIIA/B or stage IV and associated with a five-year survival of 10% orless. According to recent data from the SEER (NCI Surveillance Epidemiol-ogy and End Result Program), only 15% of lung cancers are localized at the
TABLE 1 Cancer Statistics 2003
5-Year survival
Primary site New cases (no.) Deaths (no.) 1974–76 1992–98
Lung 171,900 157,200 12 15Colorectal 147,500 57,100 50 62Breast 212,600 40,200 75 86Pancreas 30,700 30,000 3 4Prostate 220,900 28,900 67 97
108 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

time of diagnosis [1]. Currently the American Cancer Society does notrecommend screening for lung cancer, even in high-risk individuals [2].Why is screening for lung cancer not recommended? Past screening trialswith chest x-ray and sputum cytology conducted at Mayo Clinic, JohnsHopkins, and Memorial Sloan Kettering Cancer Center were unable todemonstrate a decrease in lung cancer mortality in the screened population.These National Cancer Institute sponsored studies were conducted in the1970s and 1980s and are now over twenty years old [3, 4].
It is generally agreed that stage IA/B and stage IIA/B are surgicallyresectable provided the patient has adequate pulmonary reserve and isotherwise medically fit for operation. Resection offers the best chance forcure of early stage non-small cell lung cancer [5]. The 5-year survival forpathological stage IA (T1N0M0) and IB (T2N0M0) is 67% and 57% respec-tively based on the revised international staging system for lung cancer [4].The survival with clinical stage IA and IB is less at 61% and 38%, respectively.SEER data demonstrates that the 5-year survival is 49% in patients diagnosedand treated when the disease is localized [1]. The primary problem is thattoo few patients have their lung cancer detected while it is asymptomaticand in an early stage.
Is the Chest X-ray a Good Screening Tool?
The reported frequency of missed diagnoses has varied significantly inthe literature. In one report from the Mayo Lung Project, 90% of peripheralcarcinomas (45 of 50) were visible in retrospect despite the fact that threephysicians (radiologists and pulmonologists) had prospectively reviewed allchest x-rays [6]. Twenty-seven had been visible for one year or less, but fourhad been visible for more than 2 years (patient in the screening trial hadchest films every 4 months). Seventy-five percent of the perihilar carcinomaswere visible in retrospect (12 of 16). Similarly, two-thirds of the lung cancerwere visible in retrospect in the Memorial Sloan Kettering Cancer Centerscreening trial conducted simultaneously with the Mayo Clinic Trial [7].Austin and colleagues, from New York City, reported on 27 lung cancers thatwere missed on previous chest radiographs [8]. The mean diameter of themissed lesion was 1.6 cm. Five cancers (19%) were less than one centimeter,thirteen (50%) were 1.0–1.9 cm and 5 (19%) were 2.0–2.9 cm at the timethey were missed. The interval to eventual diagnosis was 10 months with arange of 0.2 to 47 months. Quekel and associates reviewed all cases of pro-ven lung cancer from 1992–1995 from a large teaching hospital in TheNetherlands [9]. The lesion was missed, in retrospect, in 49 (19%) of 259patients with NSCLC presenting as a nodular lesion. The median diameterof the lesion missed on chest x-ray was 1.6 cm (range 0.6–3.8). The mediandelay of the missed lesion was 475 days (range 7–3, 233). The miss rate for
Poster Abstracts 109
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

lesions �10 mm was 71%; between 10–20 mm the miss rate was 29% and24% for lesions 21–30 mm. A major reason for the limitation of detectinglung lesions is that 25% of the lung parenchyma on the standard PA chestradiograph is hidden by normal structures such as the heart, mediastinum,spine and diaphragm [10]. In summary, these studies make it very clear thata chest x-ray is not a sensitive tool for detecting early lung cancer, especiallythose under 2 cm in size.
Spiral CT and Lung Cancer Detection
Recent screening reports using low dose spiral CT scans have uniformlyshown that CT detects many lung cancers that are not visible by chest roent-genogram [11, 12]. Henschke et al. detected 27 lung cancers by spiral CT,but only 7 of these were visible on chest x-ray obtained at the same time[12]. Sone and colleagues from Japan detected 44 lung cancers by screeningCT scan and only 11 were visible by chest x-ray. The chest radiograph failedto detect 79% of the lung cancers that were � 2 cm [13]. Thus, CT is farbetter at detecting smaller and presymptomatic lung cancers.
Numerous reports, from 3 different continents, have shown that spiralCT screening is capable of detecting a large percentage of lung cancerswhile they are stage I [11–16]. The ELCAP trial from New York reported that22 of 27 lung cancers detected by spiral CT screening were stage IA [12].Sobue and colleagues from Japan observed that 71% of prevalence cancersand 82% of incidence cancers were stage IA at the time of diagnosis [14].A trial from Germany reported prevalence data only and noted that 58%of the lung cancer detected were stage I [15]. These screening trials andthe Mayo Clinic trial (summarized below), have shown a substantially higherrate of detection of localized lung cancer as compared to the SEER data(current practice in the USA) which documented that only 15% of all lungcancers are localized at the time of diagnosis. Thus, spiral CT scan screeningis the most promising tool currently available that offers a substantial chanceof detecting lung cancer at a smaller size and improving survival anddecreasing lung cancer mortality.
Mayo Clinic Spiral CT Screening Trial
In 1999, Mayo Clinic investigators initiated a low dose spiral CT scanscreening trial [16]. The eligibility criteria included age �50 years, eithergender, current or former smoker (quit �10 years) of �20 pack yearsand a life expectancy of 5 years. Patients could have no history of priormalignancy within 5 years and could not be on supplemental oxygen. SpiralCT scanning was performed in a General Electric Light-Speed multi-detector scanner (4 detectors) using 5 mm collimation with 3.75 mm
110 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

reconstruction intervals, high speed mode, and radiation settings of120 kVp, 40 mA. Most patients also provided an induced sputum for cytology,and a blood sample for research purposes.
From January through December 1999, we enrolled 1520 participants.The mean age was 59 years (range 50–85). Sixty-one percent were currentsmokers and 39% former smokers. The median pack years of smoking was51 (range 20–230). The rate of yearly follow-up CT scans has been excellent(�95%).
The results through 2001 (baseline plus 2 yearly follow-up scans) havebeen reported and are summarized here [16]. There have been 40 primarylung cancers diagnosed (27 prevalence, 11 incidence, and 2 interval cancerswhich developed between screening tests). Thirty-six of the 40 lung cancerswere detected by screening spiral CT scans, 2 by sputum cytology alone, and2 interval cancers. The mean size of the detected cancer was 15 mm. Thecell types and stages of the cancers are shown in Tables 2 and 3, respectively.Of note is the fact that 25 of 36 non-small cell lung cancers had stage IA/IBdisease. Most of the cancers in this report were prevalence cancers (presenton the baseline screen). For screening to be effective one would expect ahigh proportion of incidence detected cancers to be early stage. In 2002and 2003 we have detected a substantial number of additional cancers, mostof which are incidence cancers. These will be reported at a later time.
On the baseline spiral CT scan, 782 of 1520 (51%) of the participantshad at least one uncalcified nodule that required periodic follow-up withCT scans. After 3 scans (baseline plus 2 incidence scans), 1049 of the1520 participants (69%) have had at least one nodule identified (total of2,832 uncalcified nodules). The nodules were distributed in size as shownin Table 4. A manuscript is in preparation on 3-year follow-up of all of thebaseline nodules. If a baseline nodule was less than 10 mm, then the chanceof malignancy was 0.69% [17].
Potentially curative pulmonary resections were performed in 31 of 40participants with lung cancer. There were no pneumonectomies, 26 lobec-tomies, one segmentectomy, and 4 wedge resections. Eight patients under-went removal of benign lesions, 7 wedge resections, and one lobectomy.
TABLE 2 Cell Type of Non-Small Cell Lung Cancer
Type No. %
Adenocarcinoma 20 50Bronchioloalveolar 4 10Squamous 7 18Large cell 2 5NSCLC (NOS) 3 8Small cell 4 10
Poster Abstracts 111
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

Overall, 19 participants of 1520 have died since enrollment. Five of thesedeaths were due to lung cancer, including two due to small cell.
To date we can conclude that screening spiral CT can detect early stagelung cancer at a smaller size (mean 15 mm) and earlier stage than thatobserved in standard clinical practice. No conclusions can be made aboutthe impact of screen detected cancers on lung cancer mortality. Also clearis the fact that spiral CT screening detects a high percentage of non-calcifiednodules that require follow-up. In our series only 1–2% of all detected non-calcified nodules, subsequently were proven to be cancers.
These data, and others, have paved the way for the National Lung Can-cer Screening Trial (NLST) [18]. This trial is financially supported by theNational Cancer Institute and will enroll 50,000 participants. They will berandomized to screening with low dose spiral CT scan or digital chest radio-graph. Volunteers must be 55–74 years and a current or former smoker of 30pack years. Former smokers cannot have quit more than 15 years earlier.Participants will receive a baseline plus 2 annual incidence scans, and then6–12 month telephone/mail follow-up for 5 additional years. The study has a90% power to detect a 20% difference in lung cancer mortality. Ancillarystudies to NLST will evaluate cost effectiveness and quality of life issues.A subset of participants in NLST is providing blood, sputum, and urinefor banking and subsequent testing with promising biomarkers. Approxi-mately one year after opening, the NLST trial has already accrued 35,000
TABLE 3 Stage of Lung Cancer
Prevalence n ¼ 27 Incidence n ¼ 11 Interim n ¼ 2 Total %
Stage IA 17 5 — 22 55IB 2 1 — 3 8IIA 4 — — 4 10IIB — 1 — 1 2.5IIIA 2 3 — 5 12IV — — 1 1 2.5Small cell: Limited 2 1 1 4 10
TABLE 4 Baseline Plus 2 Annual Scans �1 Uncalcified Lung Nodule
mm No. %
<4 1,735 614–7 950 338–20 136 5>20 11 1
1,049 of 1,520 participants (69%).Total of >2,832 nodules.
112 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

participants and is on schedule to complete recruitment in 18 months. Therandomization has been well accepted by participants.
Acknowledgments
Supported by the National Cancer Institute CA79935–01 and the MayoFoundation.
References
1. Jemal, A., Thomas, A., Murray, T., Samuels, A. et al. Cancer Statistics, 2003. CA Cancer J. Clin. 53:5–26, 2003.
2. Smith, R. A., Cokkinides, V., and Eyre, H. J. American Cancer Society Guidelines for Early Detectionof Cancer, 2003. CA Cancer J. Clin. 53:27–43, 2003.
3. Fontana, R. S., Sanderson, D. R., Woolner, L. B. et al. Lung Cancer Screening: The Mayo Program.J. Occup. Med. 28:746–750, 1986.
4. Melamed, M. R., Flehinger, B. J., Zaman, M. B., et al. Screening for Early Lung Cancer. Chest 86:44–53, 1984.
5. Mountain, C. F. Revisions in the International System for Staging Lung Cancer. Chest 111:1710–1717,1997.
6. Muhm, J. R., Miller, W. E., Fontana, R. S., et al. Lung Cancer Detected During a Screening ProgramUsing Four-Month Chest Radiographs. Radiology 148:609–615, 1983.
7. Heelan, R. T., Flehinger, B. J., Melamed, M. R., et al. Non-small Cell Lung Cancer: Results of the NewYork Screening Program. Radiology 151:289–293, 1984.
8. Austin, J. H. M., Romney, B. M., and Goldsmith, L. S. Missed Bronchogenic Carcinoma: RadiologicFindings in 270 Patients with a Potentially Resectable Lesion Evident in Retrospect. Radiology182:15–122, 1992.
9. Quekel, G. B. A., Kessels, A. G. H., Goei, R., and van Engelshoven, J. M. A. Miss Rate of Lung Canceron the Chest Radiograph in Clinical Practice. Chest 115:720–724, 1999.
10. Chotas, H., and Ravin, C. E. Chest Radiography: Estimated Lung Volume and Projected AreasObscured by the Heart, Mediastinum, and Diaphragm. Radiology 193:403–404, 1994.
11. Kaneko, M., Eguchi, K., Ohmatsu, H., et al. Peripheral Lung Cancer: Screening and Detection withLow-Dose Spiral CT versus Radiography. Radiology 201:698–802, 1996.
12. Henschke, C. I., McCauley, D. I., Yankelevitz, D. F., et al. Early Lung Cancer Action Project: OverallDesign and Findings from Baseline Screening. Lancet 354:99–105, 1999.
13. Sone, S., Li, F., Yang, Z. G., Takashima, S., Maruyama, Y., Hasegawa, M., Wang, J. C., Kawakami, S.,and Honda, T. Characteristics of Small Lung Concerns Invisible on Conventional Chest Radiographyand Detected by Population Based Screening Using Spiral CT. Br. J. Radiol. 73:137–145, 2000.
14. Sobue, T., Moriyama, N., Kaneko, M., et al. Screening for Lung Cancer with Low-Dose Helical Com-puted Tomography: Anti-lung Cancer Association Project. J. Clin. Oncol. 20:911–920, 2002.
15. Diederich, S., Wormanns, D., Semik, M., et al. Screening for Early Lung Cancer with Low-Dose SpiralCT: Prevalence in 817 Asymptomatic Smokers. Radiology 222:773–778, 2002.
16. Swensen, S. J., Jett, J. R., Hartman, T. E., et al. Lung Cancer Screening with CT: Mayo Clinic Experi-ence. Radiology 226:756–761, 2003.
17. Midthun, D. E., Swensen, S. J., Jett, J. R., et al. Evaluation of Nodules Detected by Screening for LungCancer with Low-Dose Spiral Computed Tomography. Am. J. Respir. Crit. Care Med. 165:A37, 2002(Abstract).
18. Aberle, D. R., Black, W. C., Goldin, J. G., et al. Experimental Design and Outcomes of the NationalLung Screening Trial (NLST): A Multicenter Randomized Controlled Trial of the Helical CT vsChest X-ray for Lung Cancer Screening. Am. J. Respir. Crit. Care Med 167:A736, 2003 (Abstract).
Poster Abstracts 113
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

Fluorescence Bronchoscopy for Early Detection ofRespiratory Cancer
S. Lam, A. McWilliams, J. LeRiche, and C. MacAulayBritish Columbia Cancer Agency, Vancouver, Canada
Although adenocarcinoma has emerged to be the predominant lungcancer cell type worldwide, in the United States and Canada, 20% to 35%of all lung cancers are still squamous cell carcinomas. In many parts of Eur-ope, up to half of all lung cancers are squamous cell carcinomas. The latterare usually located in the central airways and are difficult to detect by spiralCT in the pre-invasive and early invasive stage. Autofluorescence broncho-scopy is a sensitive method to localize small, pre-invasive and micro-invasivecancers in areas of the bronchial tree accessible by fiberoptic bronchoscopy.Autofluorescence bronchoscopy is based on differences in spectral proper-ties between normal, pre-malignant and malignant tissues when the airwaysare illuminated by violet/blue light [1]. The decrease in autofluorescenceintensity observed in pre-malignant and malignant tissues is due to a combi-nation of factors such as biochemical changes intracellularly or in the extra-cellular matrix (e.g., the amount of collagen, the redox state of NADH orflavins), architectural alterations (e.g., thickness of the epithelium) andthe microvascular density in the sub-epithelial layer. Since our original dis-covery, several commercial devices are now available for clinical use—theXillix LIFE-Lung, Storz D-Light, EFI-Wolf and the Pentax-SAFE 100 systems[1,3]. These devices vary in the number of sensors used for detection of thefluorescent light, the bandwidth of the filters in front of the sensors, and theilluminating light source. The two FDA approved systems—Xillix LIFE-Lungand the Storz D-Light, showed similar improvement in sensitivity for detect-ing high-grade dysplasia and carcinoma in-situ. A direct comparisonbetween the two devices has not been conducted.
On the average, 30% to 40% of the bronchial biopsies taken from areaswith abnormal fluorescence show benign histology or only low-grade dyspla-sia. Recent comparative genomic hybridization investigation showed thatbronchial tissue with abnormal fluorescence has frequent chromosomalaberrations while tissue with normal fluorescence does not. The natural his-tory of tissue with abnormal fluorescence but benign pathology needs to beinvestigated further.
Autofluorescence bronchoscopy plays an important role in the localiza-tion of pre-invasive and early invasive lung cancer in high-risk subjects withabnormal sputum biomarker, to detect the presence of synchronous cancerand for investigating the effect of chemopreventive agents [1,5–8]. The cur-rent limitation in applying this diagnostic method to central airway cancers is
114 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

related to the size of the fiberoptic bronchoscope. Smaller fiberoptic probesthat can reach the peripheral airways are now available. In addition, using CTas a road map, it is now possible to guide these small fiberoptic instruments toperipheral airways for diagnosis of CT detected small lung nodules using anapproach that is similar to a ‘‘Global Positioning System’’ coupled with eithera spectroscopic measurement or an actual biopsy. The ability to biopsy and tofollow the natural history of pre-invasive lesions provides a vital source ofmaterial to define more accurately what is an early lung cancer by correlatinghistopathology with the molecular profile and biology of these lesions [6,7,9].
References
1. Lam S, MacAulay C, leRiche J, Palcic B. Detection and localization of early lung cancer by fluores-cence bronchoscopy. Cancer 2000; 89(11 Suppl):2468–2473.
2. Lam S, leRiche JC, Zheng Y, Coldman A, MacAulay CE, Hawk E, Kelloff G, Gazdar AF. Sex-relateddifferences in bronchial epithelial changes associated with tobacco smoking. J Natl Cancer Inst1999; 91:691–696.
3. Beamis JF, Ernst A, Mathur P, Yung R. A multi-center study comparing autofluorescence broncho-scopy to white light bronchoscopy. Lung Cancer 2003; 41 (Suppl 2):S49.
4. McWilliams A, Mayo J, MacDonald S, leRiche J, Palcic B, Szabo E, Lam S. Lung cancer screening: adifferent paradigm. Am J Respir Crit Care Med http://ajrccm.atsjournals.org/cgi/content/abstract/200301–1440C.
5. Lam S, MacAulay C, leRiche JC, Dyachkova Y, Coldman A, Guillaud M, Hawk E, Christen MO, GazdarA. A randomized phase IIb trial of anethole dithiolethione in smokers with bronchial dysplasia. J NatlCancer Inst 2002; 94:1001–1009.
6. Venmans B, van Boxem A, Smit E, Postmus P, Sutedja T. Outcome of bronchial carcinoma in-situ.Chest 2000; 117:1572–1576.
7. Bota S, Auliac J-B, Paris C, Metayer J, Sesboue R, Nouvet G, Thiberville L. Follow-up of bronchial pre-cancerous lesions and carcinoma in-situ using fluorescence endoscopy. Am J Respir Crit Care Med2001; 164:1688–1693.
8. Thieberville L, Payne P, Metayer J, Vielkinds J, LeRiche J, Palcic B, Lam S. Molecular follow-up of apreinvasive bronchial lesion treated by 13-cis-retinoic acid. Hum Pathol 1997; 28:108–110.
9. McWilliams A, MacAulay C, Gazdar AF, Lam S. Innovative Molecular and Imaging Approaches for thedetection of lung cancer and its precursor lesions. Oncogene 2002; 21:6949–6959.
Development of Intermediate Biomarkers for PredictingLung Cancer Risk
S. A. Belinsky1 and R. E. Crowell2
1Lovelace Respiratory Research Institute, Albuquerque, NM; 2University of New Mexico,Albuquerque, NM
Transcriptional silencing by CpG island hypermethylation now rivalsgenetic changes that affect coding sequence as a critical trigger for neoplasticdevelopment and progression. Genes responsible for all aspects of normalcellular function are targeted for inactivation by methylation. Previous stu-dies by our group suggested that aberrant gene promoter methylation
Poster Abstracts 115
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

detected in sputum could serve as a molecular marker in population-basedscreening for predicting lung cancer risk. This conclusion was based onthe detection of methylation of either the p16 or O6-methylguanine-DNAmethyltransferase gene in sputum up to 3 years prior to the diagnosis of squa-mous cell carcinoma of the lung. We are now extending these promisingresults to address whether a panel of methylation markers can be developedto more accurately predict risk for lung cancer. These studies are being sup-ported through evaluation of several high-risk cohorts. A nested, case-controlstudy within the Colorado cohort is being conducted that evaluates longitud-inally the ability to detect in sputum, genes inactivated by methylation as bio-markers either alone or in combination, for predicting lung cancer risk. TheColorado cohort was established in 1992 as part of the Colorado LungSPORE and now comprises approximately 2500 subjects at high risk for lungcancer. Home collected sputum was received from the majority of partici-pants at study entry and additional longitudinal samples were collected frommany of the subjects. Approximately 100 incident cases of lung cancer haveoccurred in this cohort. We are examining in a completely blinded fashion,approximately 75 incident cases of lung cancer that were matched 1:1 byage, gender, and date of sputum collection to 75 persons who were still clinicallycancer free (controls). Methylation of the p16, O6-methylguanine-DNA methyl-transferase, death-associated protein kinase, RASSF1A, H-cadherin, PAX5 a,PAX5 b, and GATA5 genes are being assessed in sputum samples. The numberof samples collected per person ranged from one to five specimens with theaverage time from sputum sample to cancer diagnosis being 20 months.Because the Colorado cohort is comprised mainly of NonHispanic White maleswith COPD, we are establishing two additional cohorts in Albuquerque, a 2200person Veterans cohort and a 1500 person Womens cohort. Finally, recent stu-dies have also demonstrated the ability to detected aberrant methylation inDNA recovered from plasma of persons with lung cancer. We have extendedthose studies to address how methylation profiles in plasma from smokers ver-sus never smokers compare and the relationship to methylation changes seenin sputum from smokers. Interim findings from these ongoing studies will bepresented. (Supported by P50-CA-58184, CA70190, V501P-2638.)
Telomerase in Lung Cancer
J. W. ShayThe University of Texas Southwestern Medical Center at Dallas, TX
Background
Human telomeres are repetitive (TTAGGG) DNA sequences at the endof chromosomes that are progressively lost with each cell division, eventually
116 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

leading to cellular growth arrest termed replicative senescence. This cellulargrowth arrest may be produced when a telomere in a cell becomes unpro-tected eliciting a DNA damage signal. In most instances cells that accumu-late a few oncogenic mutations become senescent before they canbecome cancer cells and telomere shortening may be a potent anticancerprotection mechanism. However, almost all advanced malignancies havecells that are immortal. Maintenance of telomere stability is required forcells to escape from replicative senescence and proliferate indefinitely. Tel-omerase, a cellular ribonucleoprotein complex, is upregulated or reacti-vated in almost all human cancers [1–4] and catalyzes the synthesis andextension of telomeric repeats stabilizing telomere length. The correlationbetween telomerase activity and human tumors suggests advanced tumorgrowth almost universally requires reactivation of telomerase, and that telo-merase detection may have value in cancer diagnostics, and telomerase inhi-bitors may have efficacy in cancer therapy.
Telomerase as a Diagnostic Marker in Lung Cancer
Telomerase activity is only rarely detected at low levels in normal lung tis-sue but is detected at high levels in almost all cancer specimens. While smallcell lung carcinoma and malignant mesothelioma specimens almost univer-sally have detectable telomerase activity, there are a significant number(approximately 15%) of non-small cell carcinomas that are telomerase nega-tive [2]. In some instances primary lung carcinomas are negative or weaklypositive for telomerase activity while metastasis from the primary lesions arestrongly telomerase positive. This suggests that telomerase is not necessarilyrequired for the initiation of cancer but is likely to be required for thesustained proliferation that characterizes most advanced malignancies.Telomerase shows high sensitivity and specificity for cancer detection.
Anti-Telomerase Therapeutics
Approaches attempting to inhibit telomerase activity in cancer are justentering clinical trials and may be particularly beneficial in combinationwith standard methods for tumor burden reduction (e.g., surgery and che-motherapy). There have been several approaches to inhibit telomerase as apotential novel anticancer therapeutic modality including immunothera-pies, oncolytic viruses that target only telomerase expressing cells, and oligo-nucleotide approaches [5]. Inhibition of telomerase by targeting thefunctional RNA (hTR) template region with a 13-mer thio-phosphoramidateoligonucleotide (GRN163) results in progressive telomere shortening,growth inhibition and cell death of a variety of cancer cell lines [6–7].GRN163 in vitro has IC50 values of �0.01–0.4mM and �0.3–10mM with
Poster Abstracts 117
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

and without up-take enhancers, respectively. Although GRN163 demon-strates in vivo significant inhibitory effects on telomerase and tumor viabilitywithout up-take enhancers, even when used in the sub- to low micromolarrange, the potency has been further enhanced.
A modified N30!P50 thio-phosphoramidate oligonucleotide (GRN-140719, abbreviated GRN719) was designed and made as a second generationof telomerase inhibitor. GRN719 is complementary to the template region ofhTR (isosequential to GRN163), and it contains an additional lipid (palmi-toyl) moiety attached through an amid bond of alpha amino glycerol.GRN719 inhibits telomerase activity without the use of cellular up-take enhan-cers in a dose- and sequence-dependent manner, with IC50 values �10-foldlower than the isosequential, unmodified thio-phosphoramidate (GRN163).Even though both the match and mismatch oligonucleotides are taken upequally by cells, telomerase inhibition by both compounds is sequence speci-fic. Thus the mismatch oligonucleotide does not inhibit telomerase activityor lead to telomere shortening. Long-term treatment of the cells withGRN719 results in telomere shortening, followed by cellular senescence andapoptosis. Xenografts in nude mice can be imaged in vivo following intrave-nous injection with either FITC-labeled GRN163 or GRN719. The results sug-gest GRN719 has improved cellular uptake with increased lipophilicity asjudged by reverse phase HPLC analysis, supporting further development asan effective telomerase inhibitor for the systemic treatment of cancer.
Immortalization of Human Bronchial Epithelial Cells
Long-term cultures of normal (non cancerous) human bronchialepithelial cells (NHBEC’s) have been difficult to establish. Only a few suchcultures exist and they have been derived by over-expression of viral onco-proteins such as SV40 Large T-antigen [8]. A method to reproducibly gen-erate continuously replicating human bronchial cell lines from lungs ofpatients with and without lung cancer and with and without smokingdamage has been developed. Non-cancerous pre-immortal human bron-chial biopsies were obtained from donors undergoing surgical resectionfor lung and other cancers from areas of the lung histologically not involvedwith lung cancer. These were placed into short-term culture and thenimmortalized cell lines were generated by serial introduction of retroviralvector expressing CDK4 and hTERT, the catalytic component of human tel-omerase. We have used a similar approach for immortalizing skin keratino-cytes [9]. We were not successful in immortalizing NHBECs with hTERTalone since the culture conditions resulted in a p16-induced stress responseresulting in premature growth arrest. However, over-expression of wild typeCDK4 deregulated the cell cycle by blocking the restriction point andthe p16/RB checkpoint arrest that often occurs when culturing human
118 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

epithelial cells in plastic culture dishes in atmospheric oxygen. Combiningectopic expression of hTERT in the CDK4 expressing NHBECs preventedreplicative senescence, resulting in cultures acquiring unlimited replicativecapacity. We have now developed seven such immortalized NHBEC cultureswith most retaining a diploid karyotype. Using organotypic cultures (e.g.,growing the NHBECs on lung fibroblasts in a medium/air interface) wehave observed that the bronchial epithelial cells retain the capacity to differ-entiate. In some CDK4/hTERT expressing NHBECs there is the morpholo-gical appearance of ciliated structures and PASþ goblet cells, while in otherinstances there is histological similarities to metaplasia. Gene expressionprofiles show these cells to be distinct from commercially available bronchialepithelial cultures and from human lung cancer cells. They are being char-acterized for a variety of genetic changes known to be important in thepathogenesis of lung cancer and are being progressed to malignancy withthe introduction of defined oncogenes and/or ‘‘knockdown’’ of tumor sup-pressor genes via RNAi. In conclusion, we have established a method to rou-tinely immortalize bronchial epithelial cells that should be of great utility instudying the pathogenesis of lung cancer, in toxicology studies, and in devel-oping and testing new methods for chemoprevention.
Acknowledgments
Geron Corporation (Menlo Park, CA) and CA70907 (NCI Lung SPORE).
References
1. Kim, N.-W., Piatyszek, M.A., Prowse, K.R., Harley, C.B., West, M.D., Ho, P.L.C., Coviello, G.M., Wright,W.E., Weinrich, S.L., and Shay, J.W. Specific association of human telomerase activity with immortalcells and cancer. Science, 266:2011–2015, 1994.
2. Hiyama, K., Hiyama, E., Ishioka, S., Yamakido, M., Inai, K., Gazdar, A.F., Piatyszek, M.A., and Shay,J.W. Telomerase activity in small-cell and non-small-cell lung cancers. J. Natl. Cancer Inst., 87:895–902, 1995.
3. Shay, J.W. and Bacchetti, S. A survey of telomerase in human cancer. Eur. J. Cancer, 33:787–791, 1997.4. Bodnar, A.G., Ouellete, M., Frolkis, M., Holt, S.E., Chiu, C-P., Morin, G.B., Harley, C.B., Shay, J.W.,
Lichtsteiner, S., and Wright, W.E. Extension of lifespan by introduction of telomerase in normal hu-man cells. Science, 279:349–352, 1998.
5. Shay, J.W. and Wright, W.E. Telomerase: A target for cancer therapy. Cancer Cell, 2:257–265, 2002.6. Gryaznov, S, Pongracz, K., Matray, T., Schultz, R., Pruzan, R., Aimi, J. Chin, A., Harley, C. B.S. Herbert,
Shay, J.W., Oshima, Y., Asai, A., and Yamashita, Y. Telomerase inhibitors—oligonucleotide phosphor-amidates as potential therapeutic agents. Nucleosides, Nucleotides, and Nucleic Acids, 20:401–410, 2001.
7. Herbert, B-S., Pongracz, K. Shay, J.W., and Gryaznov, S.M. Oligonucleotide N30-P50 phosphoramidatesas efficient telomerase inhibitors Oncogene, 21:638–642, 2002.
8. Lundberg, A.S., Randell, S.H., Stewart, S.A., Elenbaas, B., Hartwell, K.A., Brooks, M.W., Fleming,M.D., Olsen, J.C., Miller, S.W., Weinberg, R.A., and Hahn, W.C. Immortalization and transformationof primary human airway epithelial cells by gene transfer Oncogene, 21:4577–4586, 2002.
9. Ramirez, R.D., Herbert, B.-S., Vaughn, M.B., Zou, Y., Gandia, K., Morales, C.P., Wright, W.E., and ShayJ.W. Bypass of telomere-dependent replicative senescence (M1) upon over expression of CDK4 innormal human epithelial cells. Oncogene, 22:433–444, 2003.
Poster Abstracts 119
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

Aerosolized Chemotherapy for Patients with Cancer
C. F. Verschraegen1, B. E. Gilbert2, and V. Knight2
1The University New Mexico Cancer Research and Treatment Center, Albuquerque, NM;2Baylor College of Medicine, Houston, TX
INTRODUCTION
Delivery of medications through the airways has been practiced for cen-turies. Medications that are delivered in current practice by the airways areantibiotics (tobramycin, pentamidine, ribavirin), and asthma medications.The intuitive thinking is to deliver a high concentration of drug at the siteof the medical illness. However, airway delivery is a method of systemic deliv-ery and intuition falls short of physiology. Because of the systemic effectobtained by inhalation, there has been an increasing trend of delivery ofdrugs by the airways [1]. A recent example is the administration of smallpeptides such as insulin, which could facilitate glucose control and compli-ance with therapy [2]. Aerosolization for cancer therapy is in its infancy.
Lung and Alveolar Physiology
Delivery of any substance to the lungs to generate a systemic absorptionis a function of the size of the substance, the anatomy of the lungs, and thephysiology of the alveoli. Lung anatomy has been well defined in the 1960’sby Weibel. The airway starts at the trachea and subsequently divides 23 timesto form the alveoli. The first 16 divisions consist of the conductive zone (tra-chea and bronchi), and the last 7 divisions form the transition and therespiratory zones, which is where absorption occurs [3]. Therefore, anyadministered substance needs to reach this last zone to be effective systemi-cally. Dosing of medication is proportional to the minute ventilation/kg ofbody weight. The total deposited dose is calculated by multiplying the aero-sol concentration of drug by the minute volume by the duration of treat-ment by the deposited fraction which is a function of the species. Becausethe size of the inhaled particle is paramount to successful delivery, formula-tion of the aerosol preparation is of extreme clinical relevance. For maximalalveolar delivery and retention, the particle size should be between 1 and 3microns MMAD (mass median aerodynamic diameter) [4].
Cancer Drugs in Development for Therapeutic Inhalation
Current research is not only aimed at deliverable pharmacologicsubstances, but also at formulations for aerosol technology. The followingcompounds are being tested for cancer therapy (Table 1).
120 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

9-Nitrocamptothecin
Our research team has been working on delivery of 9-nitrocamptothe-cin, a topoisomerase-1 inhibitor. This class of agents has the capability toeradicate human tumors in xenograft models [5]. Therefore human cancercells are extremely sensitive to camptothecin. Camptothecin analogs arenevertheless not curative in clinical settings probably because of poor distri-bution of the camptothecin lactone to the tumor cells growing in humans.We hypothesized that a modification of the formulation and a systemic deliv-ery that avoids first pass in the liver may increase the therapeutic index.Aerosol delivery of liposomal 9-nitrocamptothecin (L-9NC) may possiblydelay the opening of the lactone ring. We planned to demonstrate that deliv-ery through aerosolization of fine particles is associated with systemicabsorption and perhaps with sustained levels of closed ring 9-nitrocamp-tothecin (9-NC). Experiments in nude mice showed minimal toxicity andno weight loss, with substantial antitumor activity against breast, lung, andcolon cancer xenografts [6]. The objective of the phase I clinical trial wasto determine the feasibility and safety of administering L-9NC by aerosoliza-tion for five consecutive days per week for up to eight weeks.
Patients and Methods
Patients with primary or metastatic disease to the lungs were enrolled inthis phase I study if they fulfilled the following eligibility criteria: (1) histo-logic diagnosis of cancer, (2) failure after standard cancer treatment, (3)performance status (Zubrod PS) of 0 to 2, (4) pulmonary function >50%by spirometry and DLCO, (5) normal organ function, and (6) no sympto-matic brain metastasis.
Treatment consisted of 6.7 to 26.6mg/kg per day by aerosolizationthrough an Aerotech II nebulizer with a flow of 10 liters per minute ofair. In the feasibility cohort (cohort 1), treatment of 6.7mg/kg was givenevery day for 5 consecutive days and repeated every 3 weeks if disease
TABLE 1 Potential Candidate Drugs for Aerosol Delivery
Cytotoxics Gene therapy Biologics Formulation
Camptothecin P53 Interleukine 2 Liposomes (dilaurylphosphatidylcholine)Doxorubicin Retinoblastoma Curcumin Polyethylenimine (PEI)Paclitaxel Vitamin E derivatives AIR1 Pulmonary Drug
Delivery System (Alkermes)1
Cisplatin Retinoids Nanoparticles (Am. Bioscience, Inc.)2
GM-CSF
1http://www.alkermes.com/technologies/air.html2http://www.oncologyknowledgebase.net/oksite/nddeliverysamp.html
Poster Abstracts 121
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

remained stable. Treatments were then extended to 2, 4, 6, and 8 weeks. At 8weeks, dose escalation started. Plasma was obtained on day 4 or 5 of therapyto determine the pharmacokinetic profile of the drug in cohort 1. In theother cohorts, blood was obtained on the first and the fifth day of treatmentat specific time points. Bronchoalveolar lavages to measure the amount of9-NC were performed on consenting patients. 9-NC is absorbed systemicallyas determined by HPLC and mass spectrometry on the plasma. Disease wasevaluated by CT scan of the chest every two courses.
Results and Discussion
Twenty five patients received treatment. The mean baseline FEV1 for allpatients was 85% of predicted. Patient characteristics included a median ageof 55 years (33–84 years) and a Zubrod PS of 1 (range, 0–2). A dose-limitingtoxicity was chemical pharyngitis seen after 1 week in 2 of 2 patients at26.6mg/kg/day. At 20.0mg/kg/day, grade 2 and 3 fatigue prompting a dosereduction was seen after 4 weeks in 2 of 4 patients. Grade 2 toxic effectsincluded nausea/vomiting (9 pts), cough and bronchial irritation (6 pts),fatigue (5 pts), anemia (4 pts), neutropenia (2 pts), anorexia (1 pt), andskin rash around the face mask (1 pt). Partial remissions were observed in2 patients with endometrial cancer, and stabilization occurred in 3 patientswith primary lung cancer. 9NC was absorbed systemically. Maximum drugconcentration was seen 2 hours after the end of the aerosolization with amean concentration of 37.7 ng/ml (4 patients), falling to 6.0 ng/ml 24 hourlater (Table 2). Lactone was detected (5 ng/ml) but decreased immediatelyafter aerosolization.
Conclusions
Aerosol administration of liposomal 9NC was found to be feasible andsafe. 9NC delivered as an aerosol was detected in patient’s plasma shortly
TABLE 2 Aerosol Delivery of Liposomal 9NC—Pharmacokinetics After 1 Hour Exposure
Plasma concentration (ng/ml) from start of treatment (hr)
Patient 0:00 0:30 1:00 1:15 1:30 2:00 3:00 5:00 24:00
1 2.6 39.2 33.9 51.5 43.8 43.2 58.2 10.0 1.72 8.8 18.3 28.5 30.8 59.1 58.0 52.4 25.5 9.73 ND 7.1 13.0 13.3 12.4 13.6 15.5 10.8 4.75 2.5 7.8 10.6 14.0 18.4 19.4 18.7 16.8 1.66 10.5 21.8 26.1 36.2 42.6 54.3 28.2 23.1 12.4Mean 5.7 18.9 22.4 29.2 35.3 37.7 34.6 17.2 6.0SD 4.4 13.1 10.1 16.1 19.4 20.2 19.3 7.0 4.9Mean nM 14.5 48.0 57.1 74.2 89.7 96.0 88.0 43.9 15.3
122 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

after the start of treatment. The recommended dose for phase II studies is13.3mg/kg/day, which constitutes two consecutive 30 min nebulizationsper day from a nebulizer reservoir with 4 mg of 9NC in 10 ml of sterile water,Monday to Friday for 8 weeks every 10 weeks.
Acknowledgments
This work was supported in part by The Clayton Foundation forResearch and SuperGen, Inc.
References
1. Ramsey BW, Dorkin HL, Eisenberg JD, Gibson RL, Harwood IR, Kravitz RM, Schidlow DV, WilmottRW, Astley SJ, McBurnie MA (1993) Efficacy of aerosolized tobramycin in patients with cystic fibrosis.[see comments.]. New Eng. J. Med. 328:1740.
2. Skyler JS, Cefalu WT, Kourides IA, Landschulz WH, Balagtas CC, Cheng SL, Gelfand RA (2001) Effi-cacy of inhaled human insulin in type 1 diabetes mellitus: a randomised proof-of-concept study [seecomments]. Lancet 357:331.
3. Weibel E (1963) Geometry and dimensions of airways of conductive and transitory zones. In:E Weibel (ed) Morphometry of the Human Lung. NY: Academic Press, Inc., 110.
4. Gilbert BE, Six HR, Wilson SZ, Wyde PR, Knight V (1988) Small particle aerosols of enviroxime-con-taining liposomes. Antiviral Res. 9:355.
5. Giovanella BC, Hinz HR, Kozielski AJ, Stehlin JS, Jr., Silber R, Potmesil M (1991) Complete growthinhibition of human cancer xenografts in nude mice by treatment with 20-(S)-camptothecin. CancerRes. 51:3052.
6. Koshkina N, Waldrep J, Gilbert B (1998) Anticancer effect of liposomal 9-nitrocamptothecin aerosoldelivery on human cancer xenograft models. Proc. Am. Assoc. Cancer Res. 39:A1902.
Gene Promoter Hypermethylation in AdC from Smokers and NeverSmokers
K. K. Divine1, S. A. Belinsky1, F. Gillil2, T. Kang2, T. Bocklage3, T. Coons4,and A. Schwartz5
1Lovelace Respiratory Research Institute, Albuquerque, NM; 2University of SouthernCalifornia, Los Angeles, CA; 3University of New Mexico, Albuquerque, NM; 4St. Mary’sHospital & Medical Center, Grand Junction, CO; 5Karmanos Cancer Institute/Wayne StateUniversity School of Medicine, Detroit MI
Adenocarcinoma (AdC) is the major histological type of lung cancerdiagnosed in smokers and never smokers (NS). It is hypothesized thatAdC may arise in these two populations in part through effects on differentpathways that ultimately lead to cellular transformation and tumor forma-tion. Inactivation of critical regulatory genes by aberrant promoter hyper-methylation is a common mechanism in the development and progression
Poster Abstracts 123
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

of cancer. The purpose of this study was to investigate the frequency of aber-rant promoter methylation in tumors obtained from three different popula-tions: smokers, former uranium miners who smoked, and never smokers.Additionally, the effect of tumor location, central vs. peripheral, on geneinactivation was also examined.
Three genes, p16, death associated protein kinase (DAP-kinase) and theRas effector homologue (RASSF1A), inactivated by promoter hypermethyla-tion in lung cancer were investigated. All have critical roles in important reg-ulatory pathways. P16 was methylated in 50% (92/183), 31% (11/35), and38% (18/48) of tumors from smokers, former uranium miners and NS.The methylation frequency for DAP-kinase and RASSF1A were similar inall three groups. DAP-kinase was methylated in 36% (54/152), 32% (10/31) and 36% (17/47) while RASSF1A was methylated in 38% (59/157),41% (13/32) and 44% (20/45) of tumors from these same groups. A signif-icant linear relationship (p¼ 0.02) was observed for Dap-kinase methylationfrequency and age at diagnosis. P16 was methylated at a higher frequency incentral tumors (58%, 38/65) than in peripheral tumors (44%, 69/156). Themethylation frequency between central and peripheral tumors was not sig-nificantly different for the RASSF1A and DAP-kinase genes. Our findingsindicate that aberrant promoter hypermethylation plays a significant rolein the etiology of AdC independent of environmental exposure, sex or eth-nic differences. Additionally, these findings indicate that aberrant promotermethylation is a both a common and early event. This is demonstrated bythe fact that 92% of all tumors had at least 1 gene methylated and the multi-plicity of gene methylation was similar in stage I and stage II–IV tumors,56.4% of stage I tumors and 56.0% of stage II–IV tumors had >1 genemethylated. (Supported by NIH grants 1 F32 ES05836, ES08801 andCA60691.)
The RAR-b and FHIT Tumor Suppressor Genes Are Silencedin Murine Cancers by Methylation through Distinct Mechanisms
B. R. Vuillemenot, W. A. Palmisano, L. C. Pulling, and S. A. BelinskyLovelace Respiratory Research Institute, Albuquerque, NM
Chromosome 3p is a hotspot for allelic loss by deletion in human can-cers and contains several proposed tumor suppressor genes. The RetinoicAcid Receptor beta (RAR-b) gene, located at 3p21, encodes the primaryreceptor for retinoic acid, an important signaling molecule in lung growth,differentiation, and carcinogenesis. RAR-b has been shown to be down-regulated by methylation in human cancers. Loss of expression of the
124 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

Fragile Histidine Triad gene (FHIT), located at 3p14, has been correlatedwith promoter hypermethylation in lung, breast, and other human cancers.We have previously used lung tumors induced in mice to evaluate the timingand effect of specific carcinogen exposures on targeting genes shown to bealtered in human lung cancer. These studies were extended to characterizethe role of methylation of the RAR-b and FHIT genes in murine lung can-cers. Non-expressing cell lines displayed re-expression of both of these genesafter treatment with the demethylating agent 5-aza-20-deoxycytidine, sup-porting methylation as the inactivating mechanism. However, FHIT andRAR-b display distinct mechanisms of silencing by methylation. Approxi-mately 1600 bases of the region 50 to the translational start site of the mouseFHIT gene was sequenced following promoter walking. No CpG island wasfound. This suggests that methylation of one or a few CpG dinucleotides inkey areas, such as transcription factor binding sites, causes silencing of theFHIT gene. By contrast, RAR-b displays dense methylation of a CpG island50 to the translational start site. The most 50 area of methylation coveredan Sp1 binding site, suggesting a possible mechanism for transcriptionalsilencing. Methylation-specific PCR for RAR-b on mouse lung tumorsshowed that this gene is methylated at rates of 50–90% in cancers inducedby exposure to several different carcinogens, including cigarette smoke,NNK, methylene chloride, and vinyl carbamate. Pre-cancerous hyperplasiasshowed a frequency of RAR-b methylation of 54%. These studies indicatethat RAR-b methylation is a frequent and early event in the mouse lung can-cer pathways initiated by diverse carcinogens. Supported by ES 08801.
Characterization of the DAP-kinase Promoter and Its Modulation byCpG Island Methylation in Lung Cancer
L. C. Freeman Pulling, P. G. Marron-Terada, and S. A. BelinskyLovelace Respiratory Research Institute, Albuquerque, NM
Inactivation of the death-associated protein kinase (DAP-kinase) gene byaberrant promoter hypermethylation occurs in 20–40% of lung cancers.While loss of gene function has been clearly established, the role of gene–specific elements in methylation-induced transcriptional repression of thegene has not been characterized. Thus, the purpose of these studies wasto identify the minimal promoter region of the DAP-kinase gene and to eval-uate the effect on transcription by transfection into cell lines with an endo-genously methylated DAP-kinase gene. To map the key transcriptionalregulatory region of DAP-kinase, fragments of the promoter were amplifiedand cloned into the pGL2 vector upstream of the luciferase reporter gene.
Poster Abstracts 125
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

The DAP-kinase promoter constructs were transiently transfected intohuman lung cancer-derived cell lines that express DAP-kinase. The highestpromoter activity was detected in the fragment encompassing 1 to 71500(0 being the translational start site). The fragment 1 to 7176 accountedfor �60% of the promoter activity, thus was defined as the minimal promo-ter. Additional deletion constructs were made to further evaluate transcrip-tional regulation of the DAP-kinase promoter. These studies revealed apreviously unreported transcriptional start site and intron, and the presenceof several repressive elements located within the promoter. A 73% reductionin promoter activity was seen when the minimal promoter was transfectedinto human lung cancer cell lines with a methylated DAP-kinase gene. Bisul-fite sequencing of the minimized exogenous promoter revealed de novomethylation of only a few CpG sites. Despite the same level of de novo methy-lation present within each of the exogenous promoter fragments, a range ofrepression was observed in the different cell lines (37% to 96%). Future stu-dies will help elucidate the specific factors within the cell lines that areimportant in the transcriptional regulation of the DAP-kinase gene as wellthose that impact its sensitivity towards methylation-induced repression.Supported by ES08801.
Inhalation of Insulin in Dogs: Assessment of Insulin Levels
C. H. Hobbs1, A. D. Cherrington2, D. W. Neal2, D. S. Edgerton2, C. Leach3,R. Rosskamp4, and T. R. Strack5
1Lovelace Respiratory Research Institute, Albuquerque, NM; 2Vanderbilt University MedicalCenter, Nashville, TN; 3Nektar Therapeutics, San Carlos, CA; 4Aventis, Bridgewater, NJ;5Pfizer, Inc., New York, NY
Purpose
This study was conducted to determine if inhalation delivery of insulin isassociated with different distribution of insulin levels in the arterial, deepvenous, and portal circulation compared to SC insulin.
Methods
Vascular access for measuring blood glucose and insulin was by indwel-ling catheters into the femoral artery, the portal vein, and inferior venacava of 15 Beagle dogs. Peripheral veins were catheterised for iv deliveryof somatostatin and glucose. Six dogs received SC human regular insulin
126 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

0.36 U/kg, while dry powder human insulin 1 and 2 mg was given to another3 and 6 dogs via inhalation. Glucose was continually infused to maintaineuglycaemia.
Results
Post inhalation of 1 and 2 mg, arterial insulin levels rose quickly, beforedeclining to fasting levels by 3 h. Portal levels were lower than arterial levelsat both doses. Deep venous peaks were between arterial and portal levels. Bycontrast, SC insulin was associated with a delayed and lower peak arterialconcentration that returned to baseline after 6 h.
Conclusion
Arterial and venous levels were similar following inhalation of insulin.After inhalation of 2 mg or SC injection of 0.36 U/kg of insulin, overall insu-lin exposures in muscle or liver were similar, but more glucose was requiredto maintain euglycaemia following inhalation of insulin.
Inhaled Diesel Engine Exhaust in the Range of Plausible HumanExposure Concentrations Exacerbates Rodent Susceptibility toa Childhood Pathogen
K. S. Harrod, J. A. Berger, M. D. Reed, and J. D. McDonaldLovelace Respiratory Research Institute, Albuquerque, NM
The role of air pollutants and engine emissions in the susceptibility torespiratory viral infections is not well understood. Respiratory syncytial virus(RSV) is the leading cause of wheezing and bronchiolitis in lungs of infantsand young children. To determine the impact of relevant levels of whole die-sel engine emissions (DEE) on RSV-induced lung disease, a strain of mousethat is resistant RSV infection was exposed to DEE before infection and mar-kers of lung inflammation, lung epithelial host defense, and homeostasiswere assessed. DEE was diluted to 30 and 200 micro-g/m3 total particulatematter (PM). The exposure atmosphere gas and PM components were char-acterized in detail, and the composition was consistent with typical DEE.RSV infection did not induce notable lung histopathology, inflammation,or viral persistence in air-exposed mice, consistent with the attenuated hostresponses to RSV in C57Bl/6 mice. Prior exposure to inhaled DEE (6 h/d, 7consecutive days) prolonged the expression of RSV gene subsets, consistentwith increased lung viral burdens. Lung inflammation to RSV was increased
Poster Abstracts 127
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

in DEE-exposed mice concomitant with increased viral gene expression.Lung histopathology and specifically lung epithelial morphology were mark-edly altered during RSV infection by prior DEE exposure. Mucus productionwas increased in the airways of DEE-exposed RSV-infected mice in a concen-tration dependent manner. Immunohistochemical studies of distinct lungepithelial cell subsets indicate spatial-temporal alterations in airway epithe-lial Clara cells. These studies indicate that relevant levels of inhaled DEEexposure can exacerbate the susceptibility to RSV-induced lung diseaseand may provide a useful model for elucidating the molecular mechanismsof increased susceptibility to acute respiratory infections. (Supported byLRRI and the National Environmental Respiratory Center, a government-industry program funded by EPA, DOE, DOT, California ARB, and severalcorporations and trade associations.)
National Environmental Respiratory Center: Disentangling thePollutants Responsible for Health Effects of Complex Mixturesof Air Pollutants
J. L. Mauderly (Director)1, E. Barr1, T. Barrett1, S. Belinsky1, M. Campen1,N. Crowley1, A. Gigliotti1, K. Harrod1, J. McDonald1, M. Reed1, J. C. Seagrave1,G. Leikauf2, S. Seilkop3, and J. Swenberg4
1Lovelace Respiratory Research Institute, Albuquerque, NM; 2University of Cincinnati,Cincinnati, OH; 3SKS Consulting Services, Siler City, NC; 4University of North Carolina,Chapel Hill, NC
Air pollutants are associated statistically with a wide (and growing) rangeof adverse health effects. Although people breathe complex mixtures of aircontaminants from many natural and man-made sources, air quality regula-tions focus on a small number of individual pollutants and classes that arereviewed, debated, and studied one-at-a-time. There is growing recognitionthat the ‘‘single-pollutant’’ approach will never provide an adequate under-standing of the relationship between air quality and health, and especially aslevels of regulated air pollutants fall. Because few pollutants are measuredroutinely, epidemiologists have little ability to examine the effects of com-plex mixtures. Laboratory studies treating complex mixtures as single enti-ties (e.g., diesel emissions) shed little light on the importance of theindividual constituents. Factorial studies of combinations of individual pollu-tants cannot address permutations of more than a few pollutants. TheNational Environmental Respiratory Center (NERC, www.nercenter.org)was developed as a government-industry research program to attack themulti-pollutant problem directly. NERC focuses on a key roadblock—the
128 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

absence of an exposure-response database of sufficient breadth and detail topermit novel statistical analyses of the contributions of individual com-pounds and classes to the health effects of the mixtures. NERC is buildinga composition-response database by conducting identically-designed labora-tory dose-response inhalation studies of common source emissions havingoverlapping, but different, composition. Unusually detailed data are gener-ated on both the composition and health response axes of the data matrix.The physical-chemical components of the complex exposure atmospheresare speciated to the fullest extent possible. Health assays involving severalanimal models include general indicators of toxicity (body and organweight, clinical signs, hematology, clinical chemistry, histopathology),bronchoalveolar lavage, serum clotting factors, cardiac electrophysiology,respiratory immune responses, resistance to viral and bacterial respiratoryinfections, DNA methylation and oxidative damage, micronuclei in circulat-ing erythrocytes, and tumorigenesis in A/J mice. The first product of thestrategy will be direct comparisons of key regulated emissions (e.g., dieseland gasoline emissions, wood smoke, coal emissions, street dust, cookingfumes). The ultimate product of the strategy will be analyses of the com-bined data from all studies to reveal the key air contaminants and combina-tions driving the different health effects across all mixtures. (Supportedjointly by EPA, DOE, DOT, California ARB, and numerous corporationsand trade associations.)
Epigallocatechin Galleate Reduces Cigarette Smoke-Induced LungInflammation
J. C. Seagrave and T. MarchLovelace Respiratory Research Institute, Albuquerque, NM
Rationale
The detailed mechanisms by which cigarette smoking leads to the loss ofalveolar structure resulting in emphysema are not known. Proteolyticenzymes produced by inflammatory cells have been implicated, and oxidantstress is also likely to be a factor. Epigallocatechin galleate (EGCG), a majorpolyphenol constituent of tea, has both antioxidant and anti-proteolyticproperties. In this study, we examined the ability of EGCG to inhibit inflam-mation and lung damage in a mouse model of cigarette smoke (CS)-inducedemphysema.
Poster Abstracts 129
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.

Methods
A/J female mice were exposed to filtered air or CS at a concentration of250 mg total particulate material/m3, 6 h/day, 5 d/wk, for 16 wk. Each con-dition was tested with or without EGCG (0.005%) provided in the animals’drinking water. At the end of the exposures, the animals were euthanized bypentobarbital overdose. Bronchoalveolar lavage was performed on 6 ani-mals/group. Total and differential cell counts were performed, and totalprotein, lactate dehydrogenase (LDH), alkaline phosphatase (AP), andmetalloproteinase (MMP) activity were measured in the cell-free lavagefluid. Eight additional animals were euthanized but not lavaged. The lungsof these animals were fixed at constant pressure for determination of lungvolume, histopathology, and morphometry.
Results
CS exposure caused large increases in total cells, neutrophils, macro-phages, lymphocytes, protein, LDH, AP, MMPs, and lung weight andvolume. In CS-exposed animals, EGCG reduced lavage total cells, neutro-phils, macrophages, protein, and LDH, but had minimal effects on theMMPs or lung weight and volume.
Conclusions
EGCG effectively reduced the effects of CS on some aspects of inflamma-tion and lung damage, but did not appear to reduce the CS-induced lungvolume expansion. (Supported by NM Tobacco Settlement Funds.)
130 Poster Abstracts
Exp
Lun
g R
es D
ownl
oade
d fr
om in
form
ahea
lthca
re.c
om b
y M
cMas
ter
Uni
vers
ity o
n 10
/30/
14Fo
r pe
rson
al u
se o
nly.