ChemInform Abstract: Stereoselectivity of Triplet Photocycloadditions. Part 10. Oxazole—Carbonyl...
1
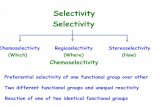
2000 oxazole derivatives oxazole derivatives R 0220 31 - 132 Stereoselectivity of Triplet Photocycloadditions. Part 10. Oxazole– Carbonyl Photocycloadditions: Selectivity Pattern and Synthetic Route to erythro α-Amino, β-Hydroxy Ketones. — The photocycload- dition of aliphatic and aromatic aldehydes (IIa)–(IIc) with oxazole (I) proceeds highly regio- and stereoselectively to give bicyclic oxetanes (III). Hydrolytic cleavage of these adducts gives selectively erythro α-amino, β-hydroxy methyl ketones. — (GRIESBECK, AXEL G.; FIEGE, MAREN; LEX, JOHANN; Chem. Commun. (Cambridge) (2000) 7, 589-590; Inst. Org. Chem., Univ. Koeln, D-50939 Koeln, Germany; EN) 1
-
Upload
axel-g-griesbeck -
Category
Documents
-
view
212 -
download
0
Transcript of ChemInform Abstract: Stereoselectivity of Triplet Photocycloadditions. Part 10. Oxazole—Carbonyl...
2000 oxazole derivatives
oxazole derivativesR 0220
31 - 132Stereoselectivity of Triplet Photocycloadditions. Part 10. Oxazole–Carbonyl Photocycloadditions: Selectivity Pattern and SyntheticRoute to erythro α-Amino, β-Hydroxy Ketones. — The photocycload-dition of aliphatic and aromatic aldehydes (IIa)–(IIc) with oxazole (I) proceedshighly regio- and stereoselectively to give bicyclic oxetanes (III). Hydrolyticcleavage of these adducts gives selectively erythro α-amino, β-hydroxy methylketones. — (GRIESBECK, AXEL G.; FIEGE, MAREN; LEX, JOHANN;Chem. Commun. (Cambridge) (2000) 7, 589-590; Inst. Org. Chem., Univ.Koeln, D-50939 Koeln, Germany; EN)
1